Natalia Dassi1, Fernanda Lagares Xavier Peres1, Meriene Viquetti de Souza2, Roberta Alves da Silva3, Marina de Almeida Furlanetto3, Adriano Nori Taniguchi2, Liane Esteves Daudt2, Mariana Bohns Michalowski2.
1Universidade Federal de Ciencias da Saude de Porto Alegre, Rio Grande do Sul, Brazil,
2Division of Hematology, Hospital de Clinicas de Porto Alegre, Rio Grande do Sul, Brazil,
3Santa Casa de Misericordia de Porto Alegre - Hospital da Criança Santo Antonio, Rio Grande do Sul, Brazil.
ADDRESS FOR CORRESPONDENCE
Dr Mariana Bohns Michalowski, Division of Hematology, Hospital de Clínicas de Porto Alegre, Ramiro Barcelos Street, 2350 - Santa Cecilia, Porto Alegre, Rio Grande do Sul, Brazil.
Email: mmichalowski@hcpa.edu.br | | Abstract | | Leukemia cutis is characterized by the infiltration of leukemic cells into the skin. As a rare presentation of acute myelogenous leukemia or acute lymphoblastic leukemia with marked clinical heterogeneity, the diagnosis may be difficult or delayed. We report two cases of leukemia cutis that occurred simultaneously at two different hospitals with very different presentations: a 3-year-old boy with a purplish-red lesion in the lower extremity resembling hemangioma; and a 4-year-old boy with an impetigo-like lesion on the scalp. We highlight the importance of including cutaneous leukemia in the differential diagnosis of children with unusual evolution of skin lesions. | | | | Keywords | | Case report, childhood leukemia, leukemia cutis, cutaneous lymphoma | | | | Introduction | Leukemia cutis (LC) is the infiltration of neoplastic leukocytes or their precursors into the epidermis, dermis, or subcutis, resulting in clinically detectable skin lesions. (1) It is a non-specific term used for cutaneous manifestations of any type of leukemia. (2) Given the wide range of clinical presentations and the low frequency of LC, diagnosis may be difficult. We report two patients with B-lineage acute lymphoblastic leukemia (ALL) whose first manifestation was LC.
Case 1
A 3 years-old boy, with normal growth and development, was brought to his pediatrician for evaluation of a lesion in the medial left calf. The lesion was 3 cm in diameter, the skin was hardened and purplish-red in color and had been in three months of evolution. There was no associated trauma. The initial clinical diagnosis was hemangioma. Considering the presence of a single hemangioma, no treatment was initiated, opting for an expectant management. Three months later, the boy was brought to the emergency department with fever (38.5°C), pain, swelling and redness at the site of lesion. The lesion had become double of the initial size. amoxicillin was prescribed but there was no improvement. Doppler ultrasound showed a heterogeneous, hypoechoic solid mass in the subcutaneous tissue with partially defined limits, measuring 7.5 × 5.1 × 2.6 cm, and a rich central arterial and venous network. Magnetic resonance imaging (MRI) of the left lower extremity revealed a subcutaneous lesion without involvement of muscles or bones. Bleeding foci were observed in some areas of the subcutaneous vascular network (Figure 1A/B). The findings were suggestive of hemangioma. The patient was treated with oxacillin for 10 days without improvement. Because of his unfavorable clinical course, the lesion was biopsied and the histopathology revealed high-grade non-Hodgkin lymphoma, with a proliferative index of 70%. Bone marrow biopsy revealed diffuse infiltration by blasts. A bone marrow aspirate showed 96% blasts, which had a regular shape, prominent nucleoli with loose chromatin and agranular cytoplasm, compatible with the morphologic diagnosis of acute lymphoblastic leukemia (ALL). Immunophenotyping confirmed the diagnosis of precursor B-cell ALL. Cerebrospinal fluid (CSF) findings were normal. Complete blood count (CBC) at this time showed hemoglobin (Hb) 10.4 g/dL, hematocrit (Ht) 30.4%, mean corpuscular volume (MCV) 72 fl, mean corpuscular hemoglobin (MCH) 25 pg, leukocytes 1,830/cumm (neutrophils 9.9%, lymphocytes 87.4%, and monocytes 2.7%), and platelets 262000/cumm. Treatment was started according to protocol BFM 2002, with good response to treatment.
Case 2
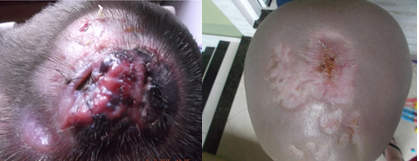
A 4 years-old boy was brought to his pediatrician due to a crusted skin lesion on the scalp. Topical and systemic antibiotic therapy was initiated for suspected impetigo. Blood counts were normal. After 3 months, as the lesion persisted, he was evaluated by a pediatric surgeon and a biopsy was performed. The results showed presence of candida spp and negative acid-fast bacilli (AFB) smears. The patient did not improve with treatment and was admitted for further investigation. The initial clinical diagnosis was pyoderma gangrenosum. Treatment was then started with prednisolone and topical silver sulfadiazine. After consultation with a dermatologist, colchicine was prescribed in combination. The lesion progressed during treatment. Two months later, he was evaluated by another dermatologist who ordered a second CBC, biopsy of the lesion, and bacterioscopic and bacteriological examination of specimens. CBC was normal. Bacteriological examination showed growth of coagulase-negative staphylococci. The anatomopathological examination revealed atypical lymphoid infiltrates of the dermis and hypodermis. Immunohistochemistry confirmed diffuse skin infiltration by B-cell ALL/lymphoma. Based on the results, the patient was referred to the pediatric hematology clinic for treatment (Figure 2A/B). His CBC on arrival showed leukocytes 73,300 cells/cumm with 36% blasts. Treatment was started according to protocol BFM 2002. The patient had poor hematologic response to 8-day corticosteroid therapy. He showed positive minimal residual disease in bone marrow aspirates on day 15 and day 33. Later he achieved complete morphologic and cytogenetic remission. The skin lesion resolved completely.
Figure 1A/B. (A) MRI of the left lower extremity; (B) Lesion appearance after antibiotic treatment and biopsy.
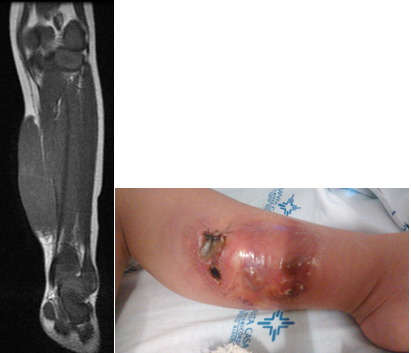
Figure 2A/B. (A) Cranial lesion after biopsy; (B) Same lesion after treatment with chemotherapy.
| | | | Discussion | The two patients reported here illustrate a rare manifestation of lymphoblastic leukemia in childhood. LC occurs most often in cases of congenital leukemia and acute myelogenous leukemia (AML). (2,3) In the pediatric population, the frequency of LC in association with AML is approximately 10%, while in association with ALL it occurs in only 1% of patients (3), with a predominance of B-cell precursors. (4) However, in most patients, this involvement is secondary to or concomitant with lymph node involvement. (5) This was not observed in the patients reported here.
The cells of acute lymphoblastic leukemia and lymphoma are immunophenotypically indistinguishable. The most important aspect of differential diagnosis lies in the amount of bone marrow involvement: greater than 20% in leukemia. The skin is involved in 33% of patients with B-cell lymphoblastic lymphoma, but in only 1% of patients with B-cell ALL. (6) In the latter case, the cutaneous involvement is known as LC.
Some case series of children with cutaneous involvement by lymphoma have been reported in the literature. Lee et al (7), in 2013, examined the clinical and histologic features of 13 patients (children and adults) with cutaneous lymphoma, six with B-cell and seven with T-cell lymphoblastic lymphoma. Most patients with B-cell lymphoma had lesions in the head and neck. (7) The same finding was previously described by Boccara et al (8).
Most cases of LC are defined once the diagnosis of systemic leukemia is established. Simultaneous involvement of these sites (bone marrow and skin) is observed in up to one-third of the cases, and LC occasionally (<10%) precedes the development of detectable leukemia in peripheral blood and/or bone marrow. (1,2,9) Therefore, a skin biopsy can be the first indication of the presence of leukemia in a subset of patients (2).
LC may have a multitude of clinical presentations. Patients may present with single or multiple skin lesions, erythematous, violaceous, or hemorrhagic papules, nodules, or infiltrated plates, generalized rash, and erythroderma. Erythematous papules and nodules are described as the most frequent lesions, what is consistent with the presentation of LC in our patients. Typically, the nodules have a firm/rubbery consistency and may become purpuric in thrombocytopenic patients. There is usually no other associated symptom. In unusual presentations, lesions may resemble guttate psoriasis, dermatitis, and stasis ulcers. There are reports of LC in herpetic lesions, intravenous catheters, and recent surgical sites. Lower extremities are the most affected body region, followed by upper limbs, back, chest, scalp, and face. (1,2,10)
LC should be distinguished from cutaneous manifestations of bone marrow dysfunction secondary to leukemic infiltration, such as petechiae, drug reactions, and infections. The differential diagnosis should also include cutaneous lymphoma (mycosis fungoides and Sézary syndrome). Mycosis fungoides is characterized by hypopigmented lesions, especially on the buttocks, while Sézary syndrome is a cutaneous lymphoma that presents as diffuse erythroderma and lymphadenopathy, similar to mycosis fungoides. Cutaneous lymphomas, however, are very rare in the pediatric population. (3)
The differential diagnosis of lymphoproliferative disorders of the skin is one of the most challenging areas in dermatopathology. Biopsy is mandatory to exclude malignant lesions in patients with therapy-resistant or unexplained skin lesions. Histopathology alone often allows the classification of cases into a particular diagnostic group (e.g., epidermotropic lymphomas), but it rarely provides a definitive diagnosis. Immunohistochemistry is essential. Performing multiple biopsies is indicated in morphologically distinct lesions, particularly in patients with suspected mycosis fungoides. (10,11)
The prognosis of B-cell ALL with cutaneous presentation seems to resemble the overall prognosis of ALL without any treatment adaptation. | | | | Conclusion | | Leukemia may present atypically as skin lesions. The two patients reported here had different cutaneous manifestations, mimicking hemangioma and infectious processes. The rarity of this presentation, along with marked clinical heterogeneity, makes clinical suspicion and accurate diagnosis difficult. We highlight the importance of performing skin biopsy of any lesion associated with unusual blood count or unexpected evolution. Biopsy allowed an accurate diagnosis in the two patients described here. | | | | Compliance with Ethical Standards | | Funding | | Financial support was provided by the Research and Event Support Fund of Hospital de Clínicas de Porto Alegre (FIPE-HCPA). | | | | Conflict of Interest None | | |
- Rao AG, Danturty I. Leukemia Cutis. Indian J Dermatol 2012; 57(6):504. [CrossRef] [PubMed] [PMC free article]
- Cho-Veja JH, Medeiros LJ, Pietro VG, Vega F. Leukemia Cutis. Am J Clin Pathol 2008; 129(1):130–142. [CrossRef]
- Sambasivan A, Keely K, Mandel K, Johnston D. Leukemia cutis: an unusual rash in a child. CMAJ 2010; 182(2):171–173. [CrossRef] [PMC free article]
- Chimenti S, Fink Puches R, Peris K, Pescarmona E, Putz B, Kerl H, Cerroni L. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol 1999; 26: 379-385. [CrossRef] [PubMed]
- Schimitt IM, Manente L, Di Matteo A, Felici F, Giangiacomi M, Chimenti S. Lymphoblastic lymphoma of the pre-B phenotype with cutaneous presentation. Dermatology. 1997; 195: 289-292. [CrossRef]
- Maitra A, McKenna RW, Weinberg AG, Schneider NR, Kroft SH. Precursor B-cell lymphoblastic lymphoma: a study of nine cases lacking blood and bone marrow involvement and review of the literature. Am J Clin Pathol. 2001; 115: 868-875. [CrossRef]
- Lee WJ, Moon HR, Won CH, Chang SE, Choi JH, Moon KC, et al. Precursor B- or T-lymphoblastic lymphoma presenting with cutaneous involvement: A series of 13 cases including 7 cases of cutaneous T-lymphoblastic lymphoma. J Am Acad Dermatol, 2014;70:318-325 [CrossRef] [PubMed]
- Boccara O, Laloum-Gynberg E, Jeudy G, Aubriot-Lorton, Vabres P, de Prost Y et al. Cutaneous B-cell lymphoblastic lymphoma in children: a rare diagnosis. J Am Academ Dermatol 2012; 66: 51-57. [CrossRef]
- Millot F, Robert A, Bertrand Y, Mechinaud F, Laureys G, Ferster A et al. Cutaneous Involvement in Children with Acute Lymphoblastic Leukemia or Lymphoblastic Lymphoma. The Children's Leukemia Cooperative Group of the European Organization of Research and Treatment of Cancer (EORTC). Pediatrics 1997; 100(1):60–64 [CrossRef] [PubMed]
- Cerroni L. Lymphoproliferative lesions of the skin. J. Clin. Pathol. 2006; 59(8):813–26. [CrossRef] [PubMed] [PMC free article]
- Serra, AMS. Abordagem Terapêutica dos Hemangiomas na Infância. Dissertação de Pós-Graduação da Faculdade de Ciências da Saúde da Universidade de Brasília. 2007. Available at URL: http://repositorio.unb.br/handle/10482/2546. Accessed on 26th January 2017.
DOI: https://doi.org/10.7199/ped.oncall.2017.26
|
| Cite this article as: | | Dassi N, Peres F L X, de Souza M V, da Silva R A, Furlanetto M d A, Taniguchi A N, Daudt L E, Michalowski M B. When Dermatitis becomes Childhood Leukemia: A Case-Based Review. Pediatr Oncall J. 2017;14: 63-65. doi: 10.7199/ped.oncall.2017.26 |
|