Sandeep Kumar, Shrikiran Aroor, Pushpa Gurudas Kini, Suneel Mundkur, Manaswita Gadiparthi.
Department of Pediatrics, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, India.
ADDRESS FOR CORRESPONDENCE
Dr Sandeep Kumar, Assistant Professor, Department of Pediatrics, Kasturba Medical College, Manipal Academy of Higher Education, Manipal. India. 576104 | | Abstract | Objective: To determine the characteristic clinical and laboratory features that help in differentiating rickettsial fever from other tropical febrile illnesses.
Methods and Material: A prospective observational study was conducted in children aged 1 month to 18 years with undifferentiated fever for 5 days admitted to the pediatric department of a tertiary care hospital from November 2015 to July 2017. Rickettsial fever was considered in those with positive Weil - Felix test with OX titer of > 1:160 (OX K for scrub typhus, OX 19 for epidemic/endemic typhus and OX 2 for spotted fever group) and/or positive IgM scrub typhus ELISA. Presenting clinical features, laboratory parameters, complications and response to therapy were analyzed.
Results: Of 324 children admitted with fever, 139 (42.9%) children were diagnosed to have rickettsial disease of which 15 children were excluded due to co-infections. Age ranged from 10 months to 17 years with a mean age of 7.2 ± 4.56 years. Common clinical manifestations were fever (100%), vomiting (46.8%), hepatosplenomegaly (39.5%), isolated splenomegaly (38.7%), pallor (35.5%), headache (31.5%), myalgia (30.6%), breathlessness (4.8%), edema (33.9%), abdominal pain (22.6%), conjunctival congestion (21%), cough (19.4%), skin rash (18.5%), eschar (17%), seizures (9.7%), altered sensorium (8.9%), and lymphadenopathy (8.9%). Complications seen were meningitis/meningoencephalitis (9%), pneumonia (5%), acute respiratory distress syndrome (ARDS) (4%), gangrene (2%), myocarditis (1.6%), acute kidney injury (AKI) (1.6%) and stroke (1.6%). Common laboratory features were elevated liver enzymes (60.5%), thrombocytopenia (54%), hypoalbuminemia (53.2%), anemia (47.6%), elevated CRP (46%), leucocytosis (41.9%), and hyponatremia (38.7%). There was no mortality and all recovered. Ninety four (76%) children were diagnosed with scrub typhus, 25 children were included in other typhus group and 5 children were diagnosed as spotted fever. Vomiting, hepatosplenomegaly, anemia, myalgia, skin rash were seen more common in patients with spotted or endemic typhus whereas eschar, seizures was seen only in children with scrub typhus. Elevated CRP, hypoalbuminemia and elevated liver enzymes were seen in all patients with spotted fever.
Conclusion: Splenomegaly is a common finding in children with rickettsial fever. Eschar is seen only in patients with scrub typhus. Elevated liver enzymes, hypoalbuminemia, leucocytosis, thrombocytopenia, elevated CRP and hyponatremia are the common laboratory features that point towards the diagnosis of rickettsial disease which is seen in all patients with spotted fever.
| | | | Keywords | | Rickettsial disease, Scrub typhus, Weil-Felix test, Hypoalbuminemia | | | | Introduction | Rickettsial diseases especially scrub typhus account for a significant portion of tropical infectious diseases prevalent in south India. Other clinically relevant typhus are epidemic/endemic typhus and spotted fever of which Indian tick typhus is seen in India. Scrub typhus is caused by Orientia tsutsugamushi with positive OX K titer in Weil Felix test. Indian tick typhus is caused by Rickettsia conori and diagnosed with positive OX 2 titer.1 The prevalence rate of scrub typhus in south India is reported to be up to 50% of children presenting with undifferentiated fever.2 Other major tropical fevers include dengue, malaria, leptospirosis and chikungunya which present as undifferentiated febrile illness. Rickettsial fever is difficult to diagnose early due to low index of suspicion and widely variable sensitive and specific serological tests.3 Common serological tests used to diagnose rickettsial fever are non-specific. The gold standard serological tests like indirect immunofluorescent antibody assay are expensive and not easily available.3,4 Most of the commonly available serological tests show significant positivity only after the first week of illness. Delay in diagnosis and initiation of appropriate treatment can result in severe complications such as acute respiratory distress syndrome (ARDS), septic shock and multisystem organ failure resulting in mortality.4 This study is done to determine the characteristic clinical and laboratory features that point towards the early diagnosis of rickettsial diseases. We also studied complications and response to pharmacotherapy in children with rickettsial diseases.
| | | | Methods & Materials | This was a prospective observational study conducted in the pediatric department of a tertiary care hospital from November 2015 to July 2017. The study population included children from 1 month to 18 years of age who presented with fever for ≥ 5 days without an identifiable infection. Subjects were considered to have rickettsial fever if they presented with one or more of the following clinical features: rash, edema, hepatosplenomegaly, lymphadenopathy or eschar and positive Weil - Felix test with OX titer of ≥ 1:160 (OX K for scrub typhus, OX 19 for other typhus group and OX 2 for spotted fever group) and/or positive IgM ELISA for scrub typhus.1,4 Children with co-infections were excluded from the study. Ethical approval was obtained from the Institutional Ethics Committee.
Base line demographic data including exposure to animals, tick bite or exposure to mites or chiggers was obtained. Investigations such as complete blood count, C-reactive protein (CRP), liver function tests, renal function tests, chest x ray, cerebrospinal fluid (CSF) examination, serum electrolytes, blood culture were performed as indicated. Serological tests to rule out other tropical febrile illnesses such as malaria, dengue, leptospirosis, enteric fever and chikungunya were also done. Serum creatine phosphokinase (CPK) levels, electrocardiography (ECG) and echocardiography were performed in children with congestive cardiac failure to rule out myocarditis. Weil Felix test was done as per manufacturer’s instructions. (OX 19, OX-2 and OX-K strains, plasma tee laboratories, Brid Fort, UK) serum dilutions from 1:20 to 1:320 were tested. OX 2, OX K or OX 19 titers of 1:160 or more were considered as significant. IgM Elisa for scrub typhus was also done in all patients using a commercial kit (In Bios international, Inc., Seattle, WA, USA). The clinical presentation, complications, treatment outcome were analysed in children with rickettsial disease.
Hypotension was defined as a systolic blood pressure below the 5th percentile for the corresponding age, sex and height. Thrombocytopenia was defined as platelet count of less than 150,000/cumm. Mild thrombocytopenia was considered with platelet count of 100,000/cumm, moderate thrombocytopenia if platelet count was between 50,000 to 100,000/cumm and severe thrombocytopenia if platelet count was <50,000/cumm. Leucopenia was defined as the total leucocyte count (TLC) of <4000/cumm, while leukocytosis was defined as TLC of >11,000/cumm.4 Acute respiratory distress syndrome (ARDS) was defined as acute onset respiratory distress with severe hypoxemia and diffuse bilateral pulmonary infiltrates on chest x ray consistent with pulmonary edema.5 Hepatitis was diagnosed when liver transaminases were >40 U/L.6 Myocarditis was diagnosed when the following conditions were observed: (i) Clinical findings consistent with left ventricular dysfunction (ii) Echocardiography – presence of global left ventricular wall motion abnormality (iii) elevated CPK-MB levels (>6.3 ng/ml) in the blood, with or without electrocardiogram (ECG) abnormalities.6,7 Acute kidney injury (AKI) was diagnosed according to the Acute Kidney Injury Network (AKIN) definition and classification where elevated serum creatinine was defined as rise by ≥0.3 mg/dL from baseline within 48 hours or rise in serum creatinine to ≥1.5 times baseline within the prior seven days.8 Hyponatremia was defined as serum sodium level less than 135 mmol/L and severe when sodium level < 125 mmol/L. CRP level of >40 mg/L was considered significant as few studies have shown CRP level of >40 mg/L was a better predictor of bacterial infection in children.9 Serum albumin <2.5 gm/dl was considered as hypoalbuminemia.10 Data were analysed using SPSS version 21. Descriptive data was expressed as percentage, median and interquartile range
| | | | Results | Out of 324 children admitted with undifferentiated fever, rickettsial disease was diagnosed in 139 (42.9%) children of whom 15 were excluded as they were simultaneously positive for other tropical febrile illnesses. Finally, 124 (38.3%) children with rickettsial disease were recruited into the study as depicted in figure 1. Remaining 185 children were diagnosed to have tropical febrile illnesses other than rickettsial disease. The age of subjects ranged from 10 months to 17 years with a mean age of 7.2 ± 4.56 years of which 82 (66.2%) children were in the age group of 6-15 years. Boys were more affected with a male: female ratio of 1.9:1. A seasonal trend was observed where maximum cases were reported between July and December indicating disease preponderance during rainy season and cooler months of the year (figure 2). History of exposure to reservoir animals was noted in 59 (47.6%) children [cattle 46 (37%), rodent 8 (6.5%), dog 5 (4%)]. History of insect bite (chigger/ticks/mite) was present in only 13 (10.5%) patients. Other environmental risk factors that were present included living in rural area close to the forest, playing near bushes and paddy fields in 42 (34%) children.
Fever was the presenting symptom in all children and 90 (72.6%) children presented with fever of >7days duration. Common symptoms and signs at presentation are described table 1. Complications are described in table 2. Common laboratory parameters are shown in table 3. Mean hemoglobin was 9.8±1.68 g/dl, mean leucocyte count was 8.16±1.17 x 109/L and mean platelet count was 117.4±107.4 x 109/L. CSF analysis showed pleocytosis in 8 out of 11 children with suspected meningitis. CSF protein and sugar were normal in all 11 children. Positive Weil Felix test result was obtained in 101 (81.4%) of children. OX-K positivity was most common (71 out of 101; 70%) followed by OX-19 (20%) and OX-2 (5%). Five children were simultaneously positive for both OX19 and OX 2 (5%). Simultaneous positivity for other OX titers was not found in those who were positive for OX-K. No child in the study group had positivity for all 3 titers. IgM scrub typhus ELISA was positive in 76 out of 124 cases (61.3%).
Total number of children diagnosed as scrub typhus were 94 (76%) of which 53 children were positive for both OX K titer and IgM scrub typhus, 18 children were positive only for OX-K titer while 23 children were positive only for IgM scrub typhus. Twenty-five children were included in other typhus group who had positive OX 19 titer with or without OX 2 positive (20 children with isolated OX 19 positivity and 5 children with simultaneous positivity for both OX 19 and OX 2). Spotted fever group included 5 children had positive only for OX 2 titer. Individual clinical and laboratory features of scrub typhus, spotted fever and other typhus are depicted in table 4. Vomiting, hepatosplenomegaly, anemia, myalgia, skin rash were seen more common in patients with spotted or endemic typhus whereas eschar, seizures was seen only in children with scrub typhus. Elevated CRP, hypoalbuminemia and elevated liver enzymes were seen in all patients with spotted fever (table 4).
Doxycycline was used to treat 114 (92%) children. Oral azithromycin was used in 5 of the children as liver enzymes were elevated and they were less than 2 years. Three children with scrub meningoencephalitis received intravenous chloramphenicol. No anti-rickettsial agent was used in the remaining 2 children as they clinically recovered before the serology report was available. Among those children treated with doxycycline, 97.7% of them showed improvement within 48 hours of starting doxycycline. Those children treated with azithromycin also became afebrile within 48 hours of initiating therapy. Duration of defervescence ranged from 18 hours to 72 hours. No patient died and all recovered.
Figure 1. Temporal profile of children with tropical febrile illness
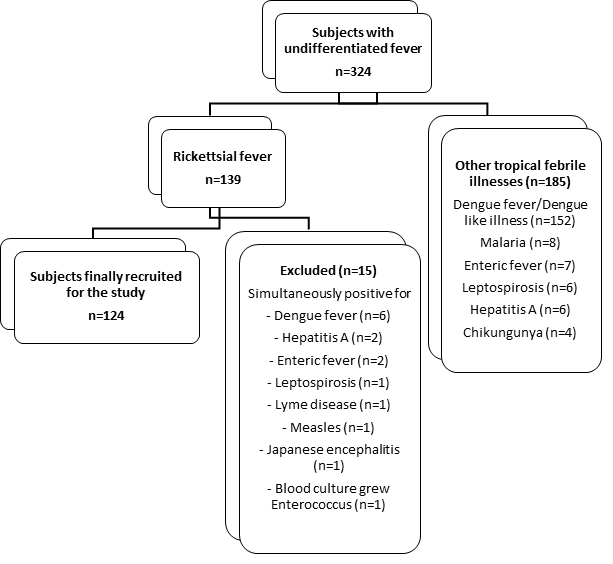
Figure 2. Line diagram demonstrating seasonal trend in case distribution
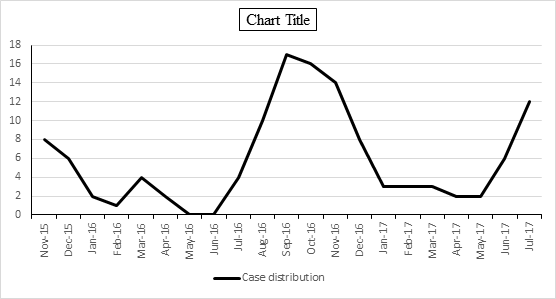
Table 1. Clinical Features of rickettsial fever at admission
| Symptoms |
Total no. of patients (n=124) (%) |
Signs |
Total no. of patients (n=124) (%) |
| Fever |
124 (100) |
Hepatosplenomegaly |
49 (39.5) |
| Vomiting |
58 (46.8) |
Isolated splenomegaly |
48 (38.7) |
| Headache |
39 (31.5) |
Pallor |
44 (35.5) |
| Myalgia |
38 (30.6) |
Edema |
42 (33.9) |
| Pain abdomen |
28 (22.6) |
Conjunctival congestion |
26 (21) |
| Cough |
24 (19.4) |
Rash |
23 (18.5) |
| Seizures |
12 (9.7) |
Eschar |
21 (17) |
| Altered sensorium |
11 (8.9) |
Isolated hepatomegaly |
19 (15.3) |
| Breathlessness |
6 (4.8) |
Tachypnea |
12 (9.7) |
| Polyarthralgia |
3 (2.4) |
Lymphadenopathy |
11 (8.9) |
| |
|
Hypotension |
5 (4) |
| |
|
Meningeal signs |
5 (4) |
| |
|
Gangrene involving extremities |
3 (2.4) |
Table 2. Complications of rickettsial diseases
| Complication |
n= 124 (%) |
| Meningitis/Meningoencephalitis |
11 (8.9) |
| Pneumonia |
6 (4.8) |
| Acute Respiratory Distress Syndrome |
5 (4) |
| Myocarditis |
2 (1.6) |
| Hepatitis |
75 (60.5) |
| Acute Kidney Injury |
2 (1.6) |
| Peripheral Gangrene |
3 (2.4) |
| Stroke |
2 (1.6) |
Table 3. Laboratory parameters of children with rickettsial disease
| Laboratory Parameter |
n= 124 (%) |
| Anemia |
59 (47.6) |
White cell count (WBC)
Leucopenia
Normal WBC count
Leucocytosis
|
8 (6.5)
64 (51.6)
52 (41.9)
|
Platelet count
Normal
Mild thrombocytopenia
Moderate thrombocytopenia
Severe thrombocytopenia
|
57 (46)
26 (21)
9 (7.2)
32 (25.8)
|
| CRP (>40mg/L) |
57 (46) |
| Hyponatremia |
48 (38.7) |
| Hypoalbuminemia |
66 (53.2) |
| Elevated liver enzymes |
75 (60.5) |
| Elevated CPK |
25 (20) |
| Elevated creatinine |
2 (1.6) |
Table 4. Clinical and laboratory features of different types of rickettsial fever
| Clinical features |
Scrub typhus group (n=94) (%) |
Other typhus group (n=25) (%) |
Spotted fever group (n=5) (%) |
P value |
| Fever |
94 (100) |
25 (100) |
5 (100) |
- |
| Vomiting |
34 (36.2) |
20 (80) |
4 (80) |
0.00015 |
| Hepatosplenomegaly |
28 (29.7) |
16 (64%) |
5 (100) |
0.00118 |
| Isolated splenomegaly |
36 (38.3) |
10 (40) |
2 (40) |
|
| Pallor |
26 (27.6) |
15 (60) |
3 (60) |
0.0055 |
| Edema |
34 (36.2) |
6 (24) |
2 (40) |
0.498 |
| Myalgia |
14 (14.9) |
20 (80) |
4 (80) |
<0.0001 |
| Conjunctival congestion |
20 (21.3) |
5 (20) |
1 (20) |
0.988 |
| Skin rash |
8 (8.5) |
12 (48) |
3 (60) |
<0.0001 |
| Eschar |
20 (21.3) |
1 (4) |
0 |
0.132 |
| Seizures |
12 (12.8) |
0 (0) |
0 |
- |
| Lymphadenopathy |
2 (2.1) |
8 (32) |
1 (20) |
0.00012 |
| Breathlessness |
6 (6.4) |
0 |
0 |
- |
| Meningeal signs |
5 (5.3) |
0 |
0 |
- |
| Gangrene |
3 (3.2) |
0 |
0 |
- |
| Polyarthralgia |
3 (3.2) |
0 |
0 |
- |
| Laboratory parameters |
|
|
|
|
| Anemia |
37 (39.4) |
18 (72) |
4 (80) |
0.0049 |
| Leucocytosis |
42 (44.7) |
7 (28) |
3 (60) |
0.228 |
| Thrombocytopenia |
46 (48.9) |
18 (72) |
3 (60) |
0.116 |
| CRP (>40mg/L) |
48 (51.1) |
4 (16) |
5 (100) |
0.002 |
| Hyponatremia |
40 (42.6) |
6 (24) |
2 (40) |
0.238 |
| Hypoalbuminemia |
51 (54.3) |
10 (40) |
5 (100) |
0.202 |
| Elevated liver enzymes |
52 (55.3) |
18 (72) |
5 (100) |
0.204 |
| Elevated CPK |
5 (5.3) |
16 (64) |
4 (80) |
<0.0001 |
| Elevated creatinine |
2 (2.1) |
0 |
0 |
- |
| | | | Discussion | Rickettsial infections are emerging tropical infectious diseases caused by obligate intracellular pleomorphic gram negative cocco-bacilli.11 Infection is acquired by transmission through arthropod vectors or by direct exposure to the reservoir animal. Common arthropod vectors that transmit the disease to humans include ticks, fleas, mites, and lice. Reservoir host animals harboring rickettsial organisms are rodents, dogs, cattle and mice.12 Spotted fever was first reported in India at Himalayan foot hills by Megaw in 1917.13 Deaths due to scrub typhus were observed during World War II era.13,14 Several reports of outbreak of rickettsial diseases are described in India.15 It is increasingly realized that rickettsial diseases are under-diagnosed in India and they significantly contribute to mortality and morbidity of tropical febrile illness. Rickettsial diseases most common being scrub typhus is increasingly reported in last 2 decades from all over India including Karnataka, Maharashtra, Tamil Nadu, Kerala, Himachal Pradesh, Delhi Haryana and North eastern states.13,15,16,17,18,19,20 Most of the outbreaks are reported between June to December suggesting predilection for cooler months of the year as seen in our study.16,17,18,19,20 Rickettsial organisms predominantly infect the vascular endothelium and reticuloendothelial cells. Vasculitis is the basic mechanism for the pathophysiology of rickettsial illness including skin rash, edema, tissue hypoxia, formation of microthrombi and end organ damage. These organisms induce various subsets of chemokine genes in infected cells, some in response to transcription factor activator protein 1 which is mainly responsible for microvascular injury.21
Rickettsial diseases account for significant cases of tropical febrile illnesses. Most of the cases initially present with fever and non-specific signs and symptoms such as vomiting, myalgia, headache and conjunctival congestion making the diagnosis difficult.4,19 The common differential diagnosis for rickettsial fever includes viral hemorrhagic fever especially dengue fever.22 Most of children diagnosed with rickettsial fever in the present study were referred to the treating physician as dengue like illness.
The skin rash in rickettsial fever is typically described as maculopapular or petechial blotchy rash usually involving the palms and soles. Rash was observed in 18.5% of our cases while the occurrence of rash in various studies ranges from 15-91% of cases.22,23,24,25 Eschar when present is a valuable sign for the diagnosis of rickettsial fever especially scrub typhus. In the present study, eschar was observed in only 17% of the cases but they were all patients with scrub typhus. Similarly, other Indian studies have reported higher occurrence of eschar in scrub typhus.26,27 Polyarthralgia involving large joints was noticed in 3 cases with no evidence of arthritis. Polyarthritis is reported by Sharma et al as an unusual manifestation of scrub typhus.28 We found combined hepato-splenomegaly in majority of cases (39.5%) while isolated hepatomegaly and splenomegaly were present in 15% and 39% of children respectively. Thus splenomegaly is a common finding in children with rickettsial illness in contrast to dengue fever where hepatomegaly is a common finding. Similar finding is reported in a scrub typhus study conducted in north India.29 In our study, vomiting, hepatosplenomegaly, anemia, myalgia, skin rash, lymphadenopathy, elevated CRP and CPK were seen in patients in spotted fever as compared to scrub typhus. Clinical evidence of fluid accumulation in the form of edema was observed in 34% of cases indicating dengue fever as the main differential diagnosis.
Most of the complications of rickettsial fever are usually seen during the second week of illness. Common complications described in the literature include severe thrombocytopenia with bleeding diathesis, meningoencephalitis, pneumonia, ARDS, dic (disseminated intravascular coagulation), AKI, myocarditis and purpura fulminans. Platelet count of <50,000/mm3 was found in 26% of our cases. Except for anicteric hepatitis found in 60% of children, other common complications observed were meningitis, ARDS, pneumonia, AKI and gangrene of the extremities. In contrast to the present study higher incidence of myocarditis (14-34%) was observed in few Indian studies.6,25 Similarly the incidence of AKI was only 2% in this study, while other studies have reported an incidence ranging from 12-23% of the subjects.20,30,31,32 It was interesting to note that 2 of the subjects presented with acute stroke in the absence of meningitis. Both cases recovered with no residual neurological deficits. Rickettsial disease presenting with cerebrovascular accident is a rare phenomenon and is attributed to cerebral vasculitis.33
Laboratory parameters that are characteristically observed in children with rickettsial fever include anemia, leucocytosis, thrombocytopenia and elevated CRP.22 Anemia was found in 47.6% of subjects while leucocytosis was observed in 42% of children. Thus anemia and leucocytosis are commonly seen in rickettsial fever in contrast to dengue fever where hemoconcentration and leukopenia are common. Elevated CRP was found in 46% of cases and in all patients with spotted fever. Raised CRP was observed in 100% of patients in a study done in Taiwan.34 Deranged liver function tests were the commonest biochemical abnormality observed in 60.5% of cases. We found hypoalbuminemia in a significant number of subjects (53.2%) with rickettsial fever. Hypoalbuminemia in a known finding in scrub typhus and is significantly associated with increased number of complications and mortality as reported in a study conducted in Republic of Korea. Endothelial damage with subsequent increased vascular permeability and extravascular protein loss is the basis of hypoalbuminemia.35 Hyponatremia is also a common finding in children with rickettsial disease.36 We found hyponatremia in 38.7% of children with rickettsial fever.
Serological evidence is the mainstay for the diagnosis of rickettsial infections. The various serological tests available include Weil Felix Test, Enzyme Linked Immuno Sorbent Assay (ELISA), Indirect Haemagglutination Test (IHA) and Indirect Immunofluorescence Antibody Test (IFA). Weil-Felix test is a heterophile agglutination test widely available for the diagnosis of rickettsial diseases even in limited resource settings.37 The gold standard serological test for the diagnosis is Indirect Immunofluorescence Antibody (IFA) test. Disadvantages of IFA are it is expensive and requires a fluorescence microscope which is not easily available in all centres. In view of the disadvantages of both IFA and Weil-Felix tests, an alternate serological test, ELISA is the most cost effective method. According to a study conducted at Himachal Pradesh, it was found that in relation to IgM ELISA, Weil-Felix test has a sensitivity and specificity of 72.5% and 91.4% respectively at a titre of 160.38 Another study done by Prakash et al, the sensitivity and specificity was 43% and 98% respectively for titres of 80 or more while the sensitivity of IgM ELISA was found to be 86.5%.39 Both these studies demonstrated higher specificity of Weil-Felix test with increasing titres. In the present study a higher titre i.e.>160 was taken as the diagnostic titre.
Rickettsial diseases respond promptly to pharmacotherapy. Similar to other studies there was a dramatic response to doxycycline in the present study. doxycycline is the drug of choice for treating rickettsial infections. Though there is controversy regarding its use in children <8 years of age, when used in appropriate dose of 2.2 mg/kg/dose twice daily for appropriate duration, adverse effects such as teeth staining, enamel hypoplasia and hepatotoxicity is relatively rare and are uncommon in children >2 years of age.40 Hence we preferred oral azithromycin in age group < 2 years. azithromycin and chloramphenicol are the other widely used anti rickettsial agents in children. chloramphenicol was used in children with meningitis as it penetrates blood-brain barrier efficiently. All children responded to pharmacotherapy along with supportive care and no mortality was observed in this study. | | | | Conclusion | | Fever for a duration of 5 days or more in a child hailing from an endemic area for rickettsial fever and history of exposure to reservoir animals may give a possible clue in suspecting rickettsial fever by history. The presence of maculopapular blotchy rash involving palms and soles, edema and hepatosplenomegaly are the characteristic features in clinical examination. Splenomegaly is a common finding in rickettsial fever in contrast to dengue fever. Eschar though present in only 17% of cases, is still a valuable clue to the diagnosis of scrub typhus. Characteristic laboratory features of rickettsial disease are anemia, leucocytosis, thrombocytopenia, elevated CRP, elevated liver enzymes, hypoalbuminemia and hyponatremia. | | | | Compliance with Ethical Standards | | Funding None | | | | Conflict of Interest None | | |
- Rathi N. Rickettsial Diseases in India- A long way ahead. Pediatr Infect Dis. 2015; 7: 61-3. [CrossRef]
- Issac R, Varghese GM, Mathai E, J M, Joseph I. Scrub Typhus: Prevalence and diagnostic issues in rural Southern India. Clin Infect Dis 2004; 39: 1395-6. [CrossRef]
- Bithu R, Kanodia V, Maheshwari RK. Possibility of scrub typhus in FUO cases: An experience from Rajasthan. Indian J Med Microbiol. 2014; 32:387-90. [CrossRef]
- Narvencar KPS, Rodrigues S, Nevrekar RP, Dias L, Dias A, Vaz M, et al. Scrub typhus in patients reporting with acute febrile illness at a tertiary health care institution in Goa. Indian J Med Res. 2012; 136:1020-4. [PMC free article]
- Kumar M, Krishnamurthy S. Scrub typhus in children at a tertiary hospital in southern India: clinical profile and complications. J Infect Public Health. 2012; 5:82–8. [CrossRef]
- Quasney MW, López-Fernández YM, Santschi M et al. The outcomes of children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015 Jun; 16 (5 Suppl 1):S118-31. [CrossRef]
- Huang CT, Chi H, Lee HC, Chiu NC, Huang FY. Scrub typhus in children in a teaching hospital in eastern Taiwan, 2000—2005. Southeast Asian J Trop Med Public Health 2009; 40:789—94. [PubMed]
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute kidney injury network. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11: R31. [CrossRef] [PMC free article]
- Putto A, Ruuskanen O, Meurman O, et al. C reactive protein in the evaluation of febrile illness. Arch Dis Child. 1986; 61:24-9. [CrossRef] [PMC free article]
- Kumar S, Aroor S, Kini GP, Mundkur S, Moideen A. Hypoalbuminemia as a marker of adverse outcome in children admitted to pediatric intensive care unit. Indian J Child Health. 2018;5(1): 6-10.
- Brooks GF, Carroll KC, Butel JS, Morse SA, Mietzner TA. Jawetz et al. Adelberg's Medical Microbiology. China: McGraw-Hill Medical; 2007. p. 349-58.
- Rathi N, Rathi A. Rickettsial infections: Indian perspective. Indian pediatr 2010; 47:157-64. [CrossRef] [PubMed]
- Padbidri VS, Gupta NP. Rickettsiosis in India: A review. J Indian Med Assoc 1978; 71:104-7. [PubMed]
- Philip CB. Tsutsugamushi disease in World War II. J Parasitol 1948; 34: 169–191. [CrossRef] [PubMed]
- Dasari V, Kaur P, Murhekar MV. Rickettsial disease outbreaks in India: A review. Ann Trop Med Public Health 2014; 7:249-54. [CrossRef]
- Kumar K, Saxena VK, Thomas TG, Lal S. Outbreak investigation of scrub Typhus in Himachal Pradesh (India). J Commun Dis 2004; 36:277-83. [PubMed]
- Sundhindra BK, Vijaykumar S, Kutti AK. Rickettsial spotted fevers in Kerala. Natl Med J India. 2004; 17:51-2. [PubMed]
- Shah V, Vaidya V, Bang V, Shah I. Spotted fever in a child in Mumbai, India. J Vector Borne Dis. 2009; 4:310-2.
- Dass R, Deka NM, Duwarah SG, Barman H, Hoque R, Mili D, et al. Characteristics of pediatric scrub typhus during an outbreak in the North Eastern Region of India: Peculiarities in clinical presentation, laboratory findings and complications. Indian J Pediatr 2011; 78:1365-70. [CrossRef] [PubMed]
- Vivekanandan M, Mani A, Priya YS, Singh AP, Jayakumar S, Purty S. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India 2010; 58:24-8. [PubMed]
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003; 16:429-36. [CrossRef] [PubMed]
- Krishna MR, Vasuki B, Nagaraju K. Scrub typhus: audit of an outbreak. Indian J Pediatr. 2015; 82:537–40. [CrossRef] [PubMed]
- Mahajan SK, Rolain JM, Sankhyan N, Kaushal RK, Raoult D. Pediatric scrub typhus in Indian Himalayas. Indian J Pediatr 2008; 75:947-9. [CrossRef] [PubMed]
- Somashekar HR, Moses PD, Pavithran S, Mathew LG, Agarwal I, Rolain JM, et al. Magnitude and features of scrub typhus and spotted fever in children in India. J Trop Pediatr 2005; 16:228-9.
- Kulkarni A, Vaidya S, Kulkarni P, Bidri LH, Padwal S. Rickettsial disease-an experience. Pediatr Infect Dis 2009; 1:118-24.
- Palanivel S, Nedunchelian K, Poovazhagi V, Raghunadan R, Ramachandran P. Clinical profile of scrub typhus in children. Indian J Pediatr 2012; 79:1459–62. [CrossRef] [PubMed]
- Ganesh R, Suresh N, Pratyusha LL, Janakiraman L, Manickam M, Andal A. Clinical profile and outcome of children with scrub typhus from Chennai, South India. Eur J Pediatr 2018; 177:887–890. [CrossRef] [PubMed]
- Sharma N, Prasad R. Polyarthritis: An Unusual Presentation of Scrub Typhus. Pediatr Oncall J. 2018;15:48. [CrossRef]
- Sharma N, Biswal M, Kumar A, Zaman K, Jain S, Bhalla A. Scrub Typhus in a Tertiary Care Hospital in North India. Am J Trop Med Hyg. 2016;95(2):447–51. [CrossRef] [PubMed] [PMC free article]
- Yen TH, Chang CT, Lin JL, Jiang JR, Lee KF. Scrub typhus: a frequently overlooked cause of acute renal failure. Ren Fail 2003; 25:397-410. [CrossRef] [PubMed]
- Kim DM, Kang DW, Kim JO, Chung JH, Kim HL, Park CY, et al. Acute renal failure due to acute tubular necrosis caused by direct invasion of Orientia tsutsugamushi. J Clin Microbiol 2008; 46:1548-50. [CrossRef] [PubMed] [PMC free article]
- Attur RP, Kuppasamy S, Bairy M, et al. Acute kidney injury in scrub typhus. Clin Exp Nephrol. 2013; 17:725-729. [CrossRef] [PubMed]
- Kumar S P, K P. Neurorickettsioses: A Rare Presentation with Stroke in a Young Adult. Journal of Clinical and Diagnostic Research: JCDR. 2014;8:MD03-MD04. doi:10.7860/JCDR/2014/9646.4996. [CrossRef]
- Huang C-T, Chi H, Lee HC, Chiu NC, Huang FY. Scrub typhus in children in a teaching hospital in eastern Taiwan, 2000–2005. Southeast Asian J Trop Med Public Health. 2009; 40:789–94. [PubMed]
- Lee CS, Min IS, Hwang JH, Kwon KS, Lee HB. Clinical significance of hypoalbuminemia in outcome of patients with scrub typhus. BMC Infect Dis 2010; 10:216-20. [CrossRef] [PubMed] [PMC free article]
- Hullatti, C, Latha G S, Babu Veeresh B V. Hyponatremia: a diagnostic marker for the diagnosis of Rickettsial diseases. International Journal of Contemporary Pediatrics 2017, 4(3), 696-699. doi:http://dx.doi.org/10.18203/2349-3291.ijcp20170993. [CrossRef]
- Mahajan SK, Kashyap R, Kanja A, Sharma V, Prasher BS, Pal LS. Prevalence of Weil- Felix test in Southern India. Clin Infect Dis. 2004; 39: 1395-1396.
- Rani S, Thakur K, Sood A et al. Comparison of weil felix test and IgM ELISA in the diagnosis of scrub typhus in Kangra, Himachal Pradesh. Int J Health Sci Res. 2016; 6:28-32.
- Prakash JA, Abraham OC, Mathai E. Evaluation of tests for serological diagnosis of scrub typhus. Trop Doct 2006; 36: 212-3. [CrossRef] [PubMed]
- Rathi N, Kulkarni A, Yewale V. IAP guidelines on rickettsial diseases in children. Indian Pediatr. 2017; 54:223–9. [CrossRef] [PubMed]
DOI: https://doi.org/10.7199/ped.oncall.2019.11
|
| Cite this article as: | | Kumar S, Aroor S, Kini P G, Mundkur S, Gadiparthi M. Clinical and Laboratory Features of Rickettsial diseases in children in South India. Pediatr Oncall J. 2019;16: 9-16. doi: 10.7199/ped.oncall.2019.11 |
|