B.S. Ramakrishna.
Department of Gastrointestinal Sciences, Christian Medical College & Hospital, Vellore 632004, India.
ADDRESS FOR CORRESPONDENCE
Prof B.S. Ramakrishna, Department of Gastrointestinal Sciences, Christian Medical College & Hospital, Vellore 632004, India.
Email: rama@cmcvellore.ac.in | Acute diarrheal disease continues to claim the lives of an estimated half a million children annually in India and of nearly two million children worldwide.(1) The global reduction in mortality from approximately 5 million diarrheal deaths annually twenty years ago, (2) is attributed to the widespread utilization of oral rehydration solution (ORS). The introduction of ors, hailed as one of the most significant medical advances of the twentieth century, allowed correction of dehydration and prevention of mortality. (3) ors use was based on the fact that cyclic AMP and other diarrhea mediators inhibited sodium chloride absorption, but not glucose-linked sodium absorption. However, conventional glucose-based ors did not reduce duration or severity of diarrhea and paradoxically increased diarrhea in some children. (4) This led to attempts to identify solutes that would stimulate sodium absorption and at the same time reduce diarrhea. Though amino acid-linked sodium absorption was another potential target for ors development, a number of studies that compared amino acid-based ors with glucose-based ors did not reveal any superiority of the former. In the 1980s, cereal-based ors was also widely tested as an alternative to the glucose-based ors. Cereal-based ors was very effective, and of these only rice-based ors survived over time. Though rice-based formulations were identified for home preparation and use, there is at least one commercially available formulation that uses rice as the glucose donor. An early meta-analysis of 13 clinical trials suggested that, compared to glucose-ORS, rice-ORS reduced stool output by about 18% in children with non-cholera diarrhea. (5) This meta-analysis also suggested that most of the effectiveness of rice- or other cereal-based ors was due to the low osmolarity of the ors used. Hypo-osmolar ors was particularly effective in the subgroup without rotavirus. Following customary practice in many parts of the world, food intake was generally withheld during diarrhea for fear of aggravating symptoms. However, in the 1980s, work primarily led by pediatricians in Bangladesh showed that early refeeding was effective in reducing diarrhea in children and this became a cornerstone of therapy. Bhan et al in 1994 compiled a summary of several attempts to improve ors for diarrhea in children and concluded that rice-based ors, maltodextrin-containing and amino acid-containing ors were not superior to glucose-based ors for acute non-cholera diarrhea, provided that feeding was promptly resumed after initial rehydration of the child. (6) Several trials of rice-based ors continued to be published, and the most recent meta-analysis concluded that rice-based ors reduced mean stool output by 51-67% in adults and children with cholera, but was not significantly better than glucose ors in children with non-cholera diarrhea, where mean stool output was only 4 ml/kg lower than glucose ors. (7)
Following the introduction of ors in the late 1960s by various groups in the Philippines, in Dhaka and in Kolkata, (8,9) the ors formulation that was approved by the World Health Organization in 1975 is shown in Table 1. This ors was developed following studies of fecal electrolyte composition in cholera, a disease that causes extreme dehydration and affects adults more often than children. Common cause of diarrhea in children such as rotavirus infection is characterized by lesser degree of fecal sodium loss. Pediatricians in Europe using the original glucose-ORS for children with diarrhea quickly recognized that use of this ors sometimes caused hypernatremia. This may have been due to the fact that the children were given only ors and not allowed supplemental water, whereas the standard practice elsewhere was to allow free intake of water in addition to ors.
Table 1. Composition of standard oral rehydration solution and new reduced osmolarity ORS as recommended by the World Health Organization.
| Ingredient | Standard WHO ORS mmol/l | Reduced osmolarity ORS mmol/l | | Glucose | 111 | 75 | | Na | 90 | 75 | | K | 20 | 20 | | Cl | 80 | 65 | | Citrate | 10 | 10 | | Osmolarity mOsm/kg | 311 | 245 |
By the 1980s, European pediatricians began to use an ors that had a lower content of sodium (usually 60 mmol/l) and lower osmolarity, and this was endorsed by the European associations. (10) At around this time, a scientific basis for reducing the osmolarity of ors also began to emerge. Since the 1960s, it was known that reducing the osmolarity of a solution that was ingested increased the absorption of water from that solution. (11) In the 1980s, attempts were made to study the physiological effects of reduced osmolarity in the secreting intestine. Rolston and others showed that reducing the osmolarity improved small intestinal water absorption from ors in animals with experimentally induced diarrhea. (12,13) Subsequently, Rolston also showed that reducing the sodium concentration of ors led to better water absorption in human volunteers in south India and would be appropriate in countries with a high prevalence of tropical enteropathy. (14)
These experimental studies were followed by a number of clinical studies in patients with diarrhea, including a multi-center international trial. (15) Meta-analysis of these studies concluded that, compared to WHO standard ors, hypo-osmolar ors was associated with fewer unscheduled intravenous fluid infusions, lower stool volumes, and less vomiting.(16,17) These differences are shown in Table 2. Based on these studies, the UNICEF and WHO organized a meeting of experts in New York in July 2001 which suggested a consensus reduced osmolarity ors formulation.(18) The WHO officially incorporated this change in the recommended composition of ors to this low sodium, low glucose, low osmolarity solution in 2002.
The Journal of the American Medical Association in 2004 carried two opinion pieces, one defending the change in ors to reduced osmolarity solution, and the other pointing out the potential problems that may be encountered with recommending a reduced osmolarity solution for universal use in all forms of diarrhea.(19,20) A meta-analysis of studies of reduced osmolarity ors in patients with cholera concluded that its use is associated with biochemical hyponatremia when compared with standard ors, although there were similar benefits in terms of outcomes; and that under wider practice conditions, where monitoring is likely to be difficult, caution is warranted in the use of reduced osmolarity ors in cholera or other severe diarrhea.(21) Therefore it may be useful to have one solution (with low sodium and low osmolarity) for use in children with diarrhea, while another solution (the old WHO formula) may be more appropriate for adults with cholera.
The use of zinc to reduce diarrhea duration has been widely studied. Zinc supplementation has been found to reduce diarrhea duration and to reduce the frequency of persistent diarrhea and consequent malnutrition which complicates diarrhea in children in developing countries.(22,23) Currently the consensus is to use zinc in the form of 20 mg elemental zinc given once daily for 14 days.
Although reduced osmolarity ors has major advantages in the treatment of children with non-cholera diarrhea, the reduction of diarrhea volume and duration is a goal that remains to be attained. Short chain fatty acids are produced in the colon by fermentation of unabsorbed carbohydrate and stimulate sodium absorption. Like glucose-linked sodium absorption in the small intestine, this absorptive process in the colon is not altered in diarrheal disease. Amylase resistant starch, found in some foods such as cereals and green banana, is fermented to short chain fatty acids in the colon and reduces diarrhea both in adults with cholera and in children with non-cholera diarrhea. (24,25)
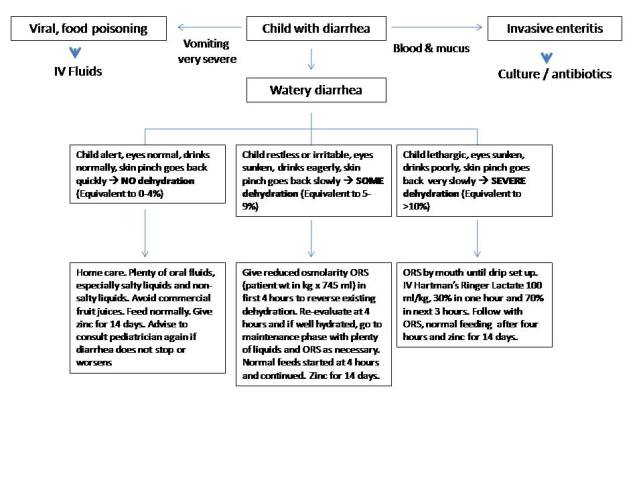
Children presenting with acute diarrhea should be assessed as to whether they have a watery diarrhea syndrome or bloody diarrhea syndrome, and whether there is evidence of clinical dehydration. Dehydration is assessed as 'none', 'some' or 'severe' according to the WHO Management of diarrhoea guidelines (Figure 1). Children who have severe dehydration require intravenous hydration (with Ringer lactate solution preferentially) of which 100 ml/kg is given over four hours, 30% in the first hour and the remaining 70% over three hours. Children with 'some' dehydration are presumed to have dehydration of between 5-10% of body weight and should be treated with reduced osmolarity ors. ors can be administered via nasogastric tube in the initial stages of rehydration in sick children who are unable to take adequate amounts of ors. It is estimated that approximately one in every 25 children that are given ors will go on to require intravenous hydration. Children are assessed frequently and oral intake, urine output and stool output measured. It is expected that rehydration (replacement of lost fluid) would have completed in the first four hours and the subsequent phase is maintenance of hydration, where ors intake is matched to stool losses. The usual diet of the child is allowed after the first four hours in children with significant dehydration, and is continued without interruption in normal in children with no dehydration. The use of zinc has become commonplace and 20 mg of elemental zinc is given as suspension or tablet once daily for 14 days. The use of starchy foods is encouraged. Commercially available fruit juices have a high osmolarity and are best avoided in the initial phase of hydration of the child. Antibiotics are generally not necessary in the treatment of diarrhea in children, except in older children with severely dehydrating diarrhea where cholera is suspected or proved by dark field microscopy of the feces, or in children with blood and mucus diarrhea due to an invasive organism such as Shigella. Early return to normal nutritional intake is highly desirable both in terms of recovery from the current episode of diarrheal illness and in prevention of persistent diarrhea and malnutrition.
Figure 1. Flow chart for ORS administration and management of diarrhea in children. The WHO classification of dehydration is shown and equivalent percentage dehydration is shown. Use the reduced osmolarity ORS for children. Zinc is given in 20 mg dose daily for a period not exceeding 14 days.
 | | | | Compliance with Ethical Standards | | Funding None | | | | Conflict of Interest None | | |
- Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease as estimated from studies published between 1992 and 2000. Bull World Health Organization 2003; 81: 197-204. [PubMed]
- Snyder JD, Merson MH. The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bull World Health Organization 1982; 60: 605-613. [PubMed]
- Editorial. Water with sugar and salt. Lancet 1978; 2: 300-301. [PubMed]
- Greenough WB 3rd. A simple solution: curing diarrhea. J Diarrhoeal Dis Res 1993; 11: 1-5. [PubMed]
- Gore SM, Fontaine O, Pierce NF. Impact of rice based oral rehydration solution on stool output and duration of diarrhoea: meta-analysis of 13 clinical trials. Br Med J 1992; 304: 287-291. [CrossRef]
- Bhan MK, Mahalanabis D, Fontaine O, Pierce NF. Clinical trials of improved oral rehydration salt formulation: a review. Bulletin of the World Health Organization 1994; 72: 945-955. [PubMed]
- Fontaine O, Gore SM, Pierce NF. Rice-based oral rehydration solution for treating diarrhoea. Cochrane Database Syst Rev 2000:2 CD001264.
- Nalin DR, Cash RA, Islam R, Molla A, Phillips RA. Oral maintenance therapy for cholera in adults. Lancet 1968: 370-373. [CrossRef]
- Pierce NF, Banwell JG, Mitra RC, Caranasos GJ, Keimowitz RI, Mondal A, Manji PM. Oral maintenance of water-electrolyte and acid-base balance in cholera: a preliminary report. Indian J Med Res 1968; 56: 640-645. [PubMed]
- Report of an ESPGAN Working Group. Recommendations for composition of an oral rehydration solution for the children of Europe. J Pediatr Gastroenterol Nutr 1992; 14: 113-115. [PubMed]
- Parsons DS, Wingate DL. The effect of osmotic gradients on fluid transfer across rat intestine in vitro. Biochemic Biophys Acta 1961; 46: 170-183. [CrossRef]
- Rolston DDK, Borodo MM, Kelly MJ, Dawson AM, Farthing MJG. Efficacy of oral rehydration solutions in a rat model of secretory diarrhea. J Pediatr Gastroenterol Nutr 1987; 6: 624-30. [CrossRef] [PubMed]
- Thillainayagam AV, Hunt JB, Farthing MJG. Enhancing clinical efficacy of oral rehydration therapy: Is low osmolality the key? Gastroenterology 1998; 114: 197-210. [CrossRef]
- Rolston DDK, Mathan VI. Jejunal and ileal glucose-stimulated water and sodium absorption in tropical enteropathy: implications for oral rehydration therapy. Digestion 1990; 46: 55-60. [CrossRef] [PubMed]
- World Health Organization. International study group on reduced osmolarity ORS solutions. Multicentre evaluation of reduced osmolarity oral rehydration salt solutions. Lancet 1995; 345: 282-285. [CrossRef]
- Hahn S, Kim Y, Garner P, Reduced osmolarity oral rehydration solution for treating dehydration due to diarrhoea in children: systematic review. Br Med J 2001; 323: 81-5. [CrossRef] [PubMed]
- Hahn S, Kim S, Garner P. Reduced osmolarity oral rehydration solution for treating dehydration caused by acute diarrhoea in children. Cochrane Database Syst Rev 2002; 1: CD002847. [CrossRef]
- Hahn S, Kim S, Garner P. Reduced osmolarity oral rehydration solution for treating dehydration caused by acute diarrhoea in children. Cochrane Database Syst Rev 2002; 1: CD002847. [CrossRef]
- Duggan C, Fontaine O, Pierce NF, Glass RI, Mahalanabis D, Alam NH, Bhan MK, Santosham M. Scientific rationale for a change in the composition of oral rehydration solution. JAMA 2004; 291: 2628-2631. [CrossRef] [PubMed]
- Nalin DR, Hirschhorn N, Greenough WB 3rd, Fuchs GJ, Cash RA. Clinical concerns about reduced osmolarity ORS solution. JAMA 2004;291:2632-2635. [CrossRef] [PubMed]
- Murphy C, Hahn S, Volmink J. Reduced osmolarity oral rehydration solutions for treating cholera. Cochrane Database of Systematic Reviews 2004; 4: CD003754. [CrossRef]
- Sazawal S, Black RE, Bhan MK, Bhandari N, Sinha A, Jalla S. Zinc supplementation in young children with acute diarrhea in India. N Engl J Med. 1995;333:839-44. [CrossRef] [PubMed]
- The Zinc Investigators' Collaborative Group. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr 2000;72:1516-1522. [CrossRef] [PubMed]
- Ramakrishna BS, Venkataraman S, Srinivasan P, Dash P, Young GP, Binder HJ. Amylase-resistant starch plus oral rehydration solution for cholera. N Engl J Med 2000;342:308-13. [CrossRef] [PubMed]
- Raghupathy P, Ramakrishna BS, Oommen SP, Ahmed MS Priyaa G, Dziura J, Young, GP, Binder HJ. Amylase-resistant starch as adjunct to oral rehydration therapy in children with diarrhea. J Pediatr Gastroenterol Nutr 2006;42:362-368. [CrossRef] [PubMed]
|
| Cite this article as: | | Ramakrishna B. ORS - Which one to use?. Pediatr Oncall J. 2009;6: 1-3. |
|