Bruna Melo1, Raquel Araújo2, Maria João Azevedo2, Rosa Amorim1, Lurdes Palhau2.
1Physical Medicine and Rehabilitation Service, Senhora da Oliveira- Guimarães Hospital, Porto, Portugal,
2Physical Medicine and Rehabilitation Service, Porto Hospital and Unversity Center, Porto, Portugal.
ADDRESS FOR CORRESPONDENCE
Bruna Melo, Rua dos Cutileiros 114, Creixomil, Porto, Portugal.
Email: brunacfmelo@gmail.com | | Abstract | | Sialorrhea is the uncontrolled leakage of saliva outside the mouth. This term commonly refers to anterior sialorrhea and should be distinguished from posterior sialorrhea, in which saliva spills through the oropharynx into the hypopharynx. It is considered pathologic beyond the age of four years. Chronic sialorrhea is seen in children with abnormal oral sensation and/or motor control and more infrequently when there is excessive production of saliva. Sialorrhea can lead to functional and clinical consequences for children, families and caregivers. In most cases, sialorrhea can be diagnosed from a history and physical examination, and additional investigation is usually unnecessary. Management of this disorder is complex and should be addressed by a multidisciplinary team, progressing from conservative to more invasive treatments. The present document intends to review and update the clinical aspects of sialorrhea in children. | | | | Keywords | | children, drooling, dysphagia, hypersalivation, sialorrhea | | | | Introduction | Sialorrhea or drooling refers to an unintentional loss of saliva from the mouth.1 Salivary "continence" occurs typically by 15-19 months, though a high number of children will continue to drool up until the age of three years, especially during meals. It is considered pathologic after four years of age.2
The overall prevalence of significant sialorrhea in childhood is 0.6%.2 The prevalence of sialorrhea in chronic neurological diseases is higher, with psychosocial impact and repercussion in quality-of-life.3 Some authors reported a prevalence of 10-58% in cerebral palsy.4
Sialorrhea can be classified as anterior and posterior; both can occur separately or simultaneously.4 Anterior sialorrhea is defined as saliva spilt from the mouth and can cause skin irritation or breakdown, and result in the need for frequent clothing changes. Besides that, anterior drooling can result in social embarrassment and can lead to isolation and low self-esteem. Posterior sialorrhea occurs when saliva spills through the oropharynx and into the hypopharynx and can lead to chronic aspiration and recurrent infections, representing greater risk to the child's health.5
Physiology of salivation:
A healthy child is estimated to produce 1-1.5 litres of saliva per day. Approximately 90% of saliva is produced by the major salivary glands: the parotid, submandibular and sublingual glands. The submandibular glands are responsible for 65-70% of total production, mainly at rest. The sublingual glands produce a small amount of thicker saliva, and the parotid glands produce about 20% of total and the saliva is more serous, as a result of stimulation during meals. The remainder is produced by minor salivatory glands.2
The secretory innervation of the salivatory glands is primarily under the control of the parasympathetic nervous system that reaches the submandibular glands through the facial nerve (chorda tympani nerve) and the parotid through the glossopharyngeal nerve.6 The postganglionic parasympathetic fibers release acetylcholine (ACh) at the nerve endings, and this neurotransmitter directly stimulates the secretion of saliva in the gland.2 The flow of saliva is enhanced by sympathetic innervation, which promotes the contraction of muscle fibres around the salivatory ducts.7
Etiology
Currently, it is widely accepted that sialorrhea is mainly due to oral-motor dysfunction and less frequently to hypersalivation.3 It is generally caused by conditions resulting in:
- Inability to retain saliva within the mouth: poor head control, inefficient labial closure, suction disorder, increased food residue, poor lip control, disorganized tongue and mandible mobility, decreased intraoral sensitivity, dental malocclusion, nasal obstruction (table 1);
- Excessive saliva production: due to local or systemic causes (table 2);
- Problems with swallowing: dysphagia, reduced frequency of spontaneous swallowing and inefficient swallowing.
Table 1. Causes of oral-motor dysfunction.
Central nervous system and muscular disorders
- “Static” generalized motor disorder (e.g., Cerebral palsy, stroke)
- Progressive motor disorder (e.g., Juvenile Parkinson's disease, Rett syndrome)
- Cranial nerve palsies (e.g., Bell's palsy, Moebius syndrome)
- Muscular disorders (e.g., Myasthenia gravis, polymyositis)
Neurodevelopmental causes
- Cognitive and awareness difficulties
- Severe learning disability
- Autism
Anatomical causes
- Adenoid/ tonsillar hypertrophy
- Macroglossia
- Dental malocclusion
- Poor lip closure
Table 2. Causes of hypersalivation.
Physiological
- Teething
- Nausea
- Foods
- Emotional stimuli
Local causes
- Oral inflammation - dental caries, gingivitis
- Infection - oral cavity infection, dental caries, peritonsillar or retropharyngeal abscess, epiglottitis
Systemic causes
- Toxin exposure - pesticides, mercury, snake poisoning, selenium, cocaine
- Medication - anticonvulsants, antipsychotics, morphine, lithium
- Gastroesophageal reflux
Assessment of sialorrhea
Ideally, assessment and management of salivary control difficulties should be carried out by multidisciplinary teams.1 When evaluating sialorrhea, it is crucial to take a thorough history and physical examination, focusing primarily on (figure 1):
- Medical assessment: age of onset, chronicity, severity, precipitating factors, associated symptoms, mental status, medications, perinatal and developmental history, past history and family history;3
- Social evaluation: any psychosocial or emotional stress should be a potential cause of sialorrhea. The psychosocial history also includes intrinsic motivation and child’s self-management skills and impact of sialorrhea on the child and family;5
- Physical examination: evaluate the level of alertness, emotional state, hydration status, head posture and vital signs. The oral cavity should be examined for caries, malocclusion, gingivitis and nasal airway obstruction.8 Tongue control, jaw stability, mouth closure, lip sealing, swallowing capacity and sensorimotor function should be evaluated.3 The physical examination also includes evaluation of respiratory status and lower airway and neurologic assessment.5
The severity and impact of sialorrhea can be evaluated through objective or subjective methods (figure 1). Objective methods include measurement of salivary flow rate, number of bibs used per day, frequency of clothing changes and drooling quotient.9 Subjective scales are useful and appropriate methods to evaluate the impact of sialorrhea on patients, family and caregivers.4 A variety of subjective scales have been described such as the Drooling Frequency and Severity Scale10 (table 3), visual analogue scales, Teacher's Drooling Scale and Drooling Impact Scale.11
Figure 1. Flow diagram for assessment and management of sialorrhea.
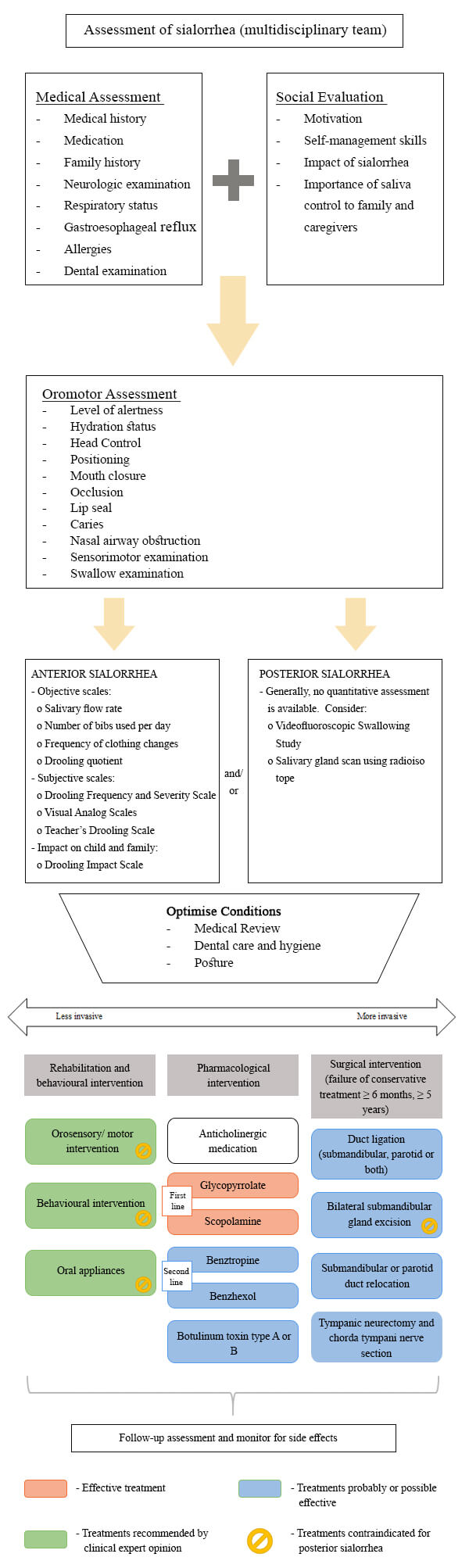
Table 3. Drooling Frequency and Severity Scale (Thomas-Stonell & Greenberg, 1988).
| Sialorrhea severity |
Points
|
| Dry (never drools) |
1 |
| Mild (wet lips only) |
2 |
| Moderate (wet lips and chin) |
3 |
| Severe (clothing becomes damp) |
4 |
| Profuse (clothing, hands, fray, objects become wet) |
5 |
| Frequency |
Points
|
| Never drools |
1 |
| Occasionally drools |
2 |
| Frequently drools |
3 |
| Constantly drools |
4 |
Additional investigation should be ordered only when indicated by a history or physical examination (figure 1). Laboratory tests are usually not necessary in most children with drooling, unless an infection is suspected. Anteroposterior and lateral radiographs of the neck using a soft tissue technique are especially useful for localising a radiopaque foreign body and detecting peritonsillar abscess, adenoid hypertrophy and swollen epiglottis.1 Ultrasound is used to diagnose local abscess.3 To rule out the possibility of gastroesophageal reflux, an oesophageal pH studies or upper gastrointestinal endoscopy may be considered.4 In case of swallowing dysfunction, a videofluoroscopic swallowing study should be done.1 An audiogram is useful to rule out conductive deafness associated with oropharyngeal conditions, that is a contraindication for tympanic neurectomy or chorda tympani nerve section, used in the treatment of sialorrhea.4 Salivary gland scan using radioisotope is indicated to determine functional status when assessing the success of surgical therapy4, but some authors suggest that these scans are mainly used for research purposes.1
Management
Several treatment strategies are available although there is no clear consensus which are the safest and most effective. The main goals in the treatment of sialorrhea are the improvement of oromotor control of secretions; enhancement of a child’s ability to behaviorally manage secretions5; reduction in social-affective and health impacts caused by sialorrhea; improved quality of life for patients and caregivers and reduction in the burden experienced by caregivers.4 A multidisciplinary team that includes paediatrician, physiatrist, neurologist, otolaryngologist, dentist and speech therapy is recommended.3 Treatment can be offered in a stepwise approach, progressing from conservative to more invasive. Complete control is often not possible.5 The methods available for the treatment of sialorrhea can be attached to the following groups (figure 1):
- Optimise conditions-Optimise positioning and medical management of factors that affect sialorrhea.5 For example, drugs that may cause or aggravate drooling should be discontinued.1
- Rehabilitation – Programs designed to improve body position and posture as well as oral motor skills may play an essential role in the management of sialorrhea. Although most studies show short term improvement with little advantage in the long run3, there is no agreement about the effectiveness of these interventions.1 Oromotor and orosensory strategies include active and passive exercises to improve lip sealing and tongue mobility, strength and positioning, sensory applications such as the use of different textures around the mouth4, vibration, manipulation like taping, palpation and biofeedback systems.3 This approach has the best chance of success if started early1 and it is only applicable to children with mild to moderate oral dysfunction, good cognitive skills and a high level of motivation.2 These techniques are easy to do in practice and do not have side effects.3
- Behavioural therapy - Children who have only moderate sialorrhea and have normal cognition may benefit from behavioural therapy.1 Behaviour modification is useful to achieve increased awareness of the mouth and its functions, frequency of swallowing and swallowing skills.3 It uses a combination of cueing, overcorrection and positive and negative reinforcement. Family members and caregivers can do this, and repeated therapy is often required. It has low-level evidence and no reported side effects.9
- Oral appliances – A variety of prosthetic devices can be beneficial such as chin cup, vestibular pads and the Innsbruck sensorimotor activator and regulator11, to achieve mandibular stability and better lip closure, tongue position and swallowing.3 Compliance can be challenging and there is some low-level evidence of their effectiveness.5 Oral devices are contraindicated in children with risk of aspirations, airway blockage and epilepsy.2
- Pharmacological treatment – As the secretion of saliva is under parasympathetic autonomic control, with ACh working as the specific neurotransmitter, downregulation of ACh would lead to a reduction in the saliva secretion.2 Systematic reviews of anticholinergic drugs show glycopyrrolate, scopolamine, benztropine and benzhexol as being effective in the treatment of sialorrhea.3 Nonetheless, pharmacological treatment with anticholinergics does not treat the underlying cause, because hypersalivation is not the main cause of sialorrhea in children.4 These drugs have adverse side-effects such as excessive thickening of secretions, urinary retention, constipation, headache, blurred vision and behavioural disturbance. There is an absolute contra-indication of using anticholinergics in individuals with glaucoma, myasthenia gravis and a history of urinary retention.2
• Glycopyronnium bromide oral – The solution formulation was approved by the FDA in 2010 for children ages 3-16 years with neurologic disorders and severe sialorrhea.13 The solution is indicated for short-term and intermittent symptomatic treatment of severe sialorrhea.14 Dosing recommendations for oral use vary among studies. The summary of product characteristics suggests a starting dose of 12.8 μg/kg/dose of glycopyrronium, three times daily. The dose can be increased every seven days to a maximum of 64 μg/kg per dose of glycopyrronium, three times daily.14 It has a quick onset of effects, within 15-30 minutes, and the peak of effect occurs within one to four hours, with a duration of action up to six to eight hours.2 It is generally better tolerated than transdermal scopolamine, with a less frequently observed but similar side effect profile. Although both medications can be used, glycopyrronium should be the first choice.15
• Transdermal scopolamine (also known as hyoscine) – scopolamine is clinically effective in treating problematic sialorrhea in children with neurodisability, especially for short-term use.15 A patch is applied behind the ear and replaced after three days, alternating sites to minimise the risk of local skin reaction.2 A paediatric drug trial evaluated dosing ranges from one quarter to one full transdermal patch.15
• Benzhexol (trihexyphenidyl) - It is used primarily for the management of dystonic movement disorders and dyskinetic cerebral palsy. In an off-label study of this drug for the treatment of dystonia or sialorrhea in children with cerebral palsy, benzhexol was generally well tolerated and effective.16 For sialorrhea, doses should start at 0.5 mg (infant) to 1 mg (child) once a day. Titrate dose every two weeks to a maximum of 2 mg three times a day. It has an onset of effect within an hour, a peak effect at one to three hours and a half-life of six to eight hours.2
• Benztropine oral - There have been a few reports that benztropine also has a positive effect on drooling.17 Dose ranges of 3-3.8 mg/day have been cited. Significant central side effects are observed, such as sedation, dysphoria and restlessness.2
• Intraglandular botulinum toxin (BTX) injections - Although BTX has many therapeutic indications, it was only in the past decade that its role for the management of drooling has been defined.8 This neurotoxin blocks release of ACh from cholinergic nerve terminals.4 The blockage is temporary as new nerve terminals sprout to create new neural connections.3 These injections are often considered after inadequate response to anticholinergic treatment.5 The effect is reported for periods between one and six months, with maximum effect at four to six weeks post-injection.2 The injection is usually given under ultrasound guidance in the parotid and submandibular glands.3 The main side effects include irritation at the injection site, dysphagia and thickening of secretions. Currently, BTX type A and B were approved by the FDA for the treatment of chronic sialorrhea in adults.18,19 In children, despite the overall positive effect of BTX injections for this purpose, the level of evidence is not sufficient to validate its efficacy.20,21
- Surgery - Many different surgical approaches have been used to treat sialorrhea. Surgical procedures should be deferred until the child is at least five years of age to allow time for complete maturation of oral motor function and coordination.8 Surgery is generally indicated in severe cases of sialorrhea, those non-responsive to six months of non-surgical therapies and when sialorrhea has a significant impact on the health and quality of life of children and family members or caregivers.1 There are several surgical techniques used to control sialorrhea: redirect the salivary flow by rerouting or relocating the salivary duct, remove the salivary glands, section the parasympathetic nerve supply to the salivary gland (e.g., chorda tympani nerve section or tympanic neurectomy) or ligate the salivary ducts (table 4). A combination of surgical approaches is usually recommended.22 Rearrangement of ducts from the submandibular glands into the tonsillar fossa and removal of the sublingual glands has become the surgical technique of choice for severe sialorrhea.4
Table 4. Advantages and disadvantages of surgical therapies for sialorrhea.
| Surgical therapy |
Advantages |
Disadvantages |
| Submandibular gland excision |
Excellent control of sialorrhea
Commonly performed procedure |
External scar
Potential for dental caries |
| Parotidectomy |
- |
Not recommended because of the risk of facial nerve injury |
| Denervation procedures |
Easy to perform, fast procedure
Does not require general anaesthesia |
It is necessary to perform bilateral tympanic neurectomies and bilateral chorda tympani nerve sections to obtain satisfactory results
Production of thick, mucoid saliva
Loss of taste to the anterior two-thirds of the tongue
Not recommended for patients with sensorineural hearing loss because this surgery is performed through the middle ear
Late failure secondary to regrowth of preganglionic fibres
|
| Ligation of the salivary ducts |
Simple and fast procedure
Decreases flow in the stimulated state |
Complications include infection in the affected glands, xerostomia and fistula formation |
| Submandibular duct relocation |
Surgical treatment of choice
Put saliva in posterior oral cavity where it can be swallowed more easily
No external scar (intraoral procedure)
The sense of taste is preserved
The amount of saliva produced is not affected
High success rate
Low morbidity
|
Potential for anterior dental caries
Potential for aspiration
Without sublingual gland excision, patient may develop ranula
|
| Parotid duct relocation |
Redirects flow in the stimulated state |
Risk of sialocele
Potential for aspiration |
| | | | Conclusion | | Sialorrhea is a significant problem for some children, especially those with neurological diseases, with significant psychosocial impact for the child and family. It is important to systematically assess the presence of sialorrhea, since posterior sialorrhea can lead to silent micro-aspirations and recurrent pneumonias, which can be life-threatening for the child. The multidisciplinary team of experts better coordinates the assessment and management of chronic sialorrhea in children. There are a considerable number of options for treatment, depending on the child's age, the severity of the problem and cognitive function.2 In general, the treatment should start with non-invasive and reversible approaches, and the surgical procedures should be reserved as the last option.9 As this problem is so significant to many children and their families, this is an area that needs future large-scale studies. | | | | Compliance with Ethical Standards | | Funding None | | | | Conflict of Interest None | | |
- Leung AK, Kao CP. Drooling in children. Paediatr Child Health. 1999;4:406-411. [CrossRef]
- Fairhurst CB, Cockerill H. Management of drooling in children. Arch Dis Child Educ Pract Ed. 2011;96:25-30. [CrossRef]
- Bavikatte G, Sit PL, Hassoon A. Management of drooling of saliva. Br J Med Pract. 2012;5: a507.
- Dias BL, Fernandes AR, Maia Filho HS. Sialorrhea in children with cerebral palsy. J Pediatr (Rio J). 2016;92:549-558. [CrossRef]
- Glader L, Delsing S, Hughes A, Parr J, Pennington L, Reddihough D et al. Sialorrhea. Bottom Line 'Evidence-Informed' Recommendations for Children/Youth with Cerebral Palsy who have sialorrhea. September 2016. (Assessed October 23, 2020). Available at: https://www.aacpdm.org/
- Myer CM, 3rd. Sialorrhea. Pediatr Clin North Am. 1989;36:1495-1500. [CrossRef]
- Hockstein NG, Samadi DS, Gendron K, Handler SD. Sialorrhea: a management challenge. Am Fam Physician. 2004;69:2628-2634.
- Little SA, Kubba H, Hussain SS. An evidence-based approach to the child who drools saliva. Clin Otolaryngol. 2009;34:236-239. [CrossRef]
- Van Hulst K, Lindeboom R, van der Burg J, Jongerius P. Accurate assessment of drooling severity with the 5-minute drooling quotient in children with developmental disabilities. Dev Med Child Neurol. 2012;54:1121-1126. [CrossRef]
- Thomas-Stonell N, Greenberg J. Three treatment approaches and clinical factors in the reduction of drooling. Dysphagia. 1988;3:73-78. [CrossRef]
- Reid SM, Johnson HM, Reddihough DS. The Drooling Impact Scale: a measure of the impact of drooling in children with developmental disabilities. Dev Med Child Neurol. 2010;52:e23-8. [CrossRef]
- Johnson HM, Reid SM, Hazard CJ, Lucas JO, Desai M, Reddihough DS. Effectiveness of the Innsbruck Sensorimotor Activator and Regulator in improving saliva control in children with cerebral palsy. Dev Med Child Neurol. 2004;46:39-45. [CrossRef]
- Eiland LS. Glycopyrrolate for chronic drooling in children. Clin Ther. 2012;34:735-42. [CrossRef]
- Glycopyrronium for severe drooling in children. Drug Ther Bull. 2017;55:93-96. [CrossRef]
- Parr JR, Todhunter E, Pennington L, Stocken D, Cadwgan J, O'Hare AE, et al. Drooling Reduction Intervention randomised trial (DRI): comparing the efficacy and acceptability of hyoscine patches and glycopyrronium liquid on drooling in children with neurodisability. Arch Dis Child. 2018;103:371-376. [CrossRef]
- Carranza-del Rio J, Clegg NJ, Moore A, Delgado MR. Use of trihexyphenidyl in children with cerebral palsy. Pediatr Neurol. 2011;44:202-206. [CrossRef]
- Camp-Bruno JA, Winsberg BG, Green-Parsons AR, Abrams JP. Efficacy of benztropine therapy for drooling. Dev Med Child Neurol. 1989;31:309-319. [CrossRef]
- Ondo W, Isaacson S, Lew M, Dashtipour K, Chary D, Clinch T, et al. RimabotulinumtoxinB (MYOBLOC) in the treatment of adult sialorrhea (P4.8-024). Neurology. 2019;92(15 Supplement): P4.8-024.
- Blitzer A, Friedman A, Michel O, Flatau-Baqué B, Csikós J, Jost W. SIAXI: IncobotulinumtoxinA for sialorrhea in Parkinson's disease, stroke, and other etiologies-Phase 3 Results (S30.007). Neurology. 2018;90(15 Supplement): S30.007.
- Porte M, Chaleat-Valayer E, Patte K, D'Anjou MC, Boulay C, Laffont I. Relevance of intraglandular injections of Botulinum toxin for the treatment of sialorrhea in children with cerebral palsy: a review. Eur J Paediatr Neurol. 2014;18:649-657. [CrossRef]
- Walshe M, Smith M, Pennington L. Interventions for drooling in children with cerebral palsy. Cochrane Database Syst Rev. 2012:CD008624. [CrossRef]
- Hussein I, Kershaw AE, Tahmassebi JF, Fayle SA. The management of drooling in children and patients with mental and physical disabilities: a literature review. Int J Paediatr Dent. 1998;8:3-11. [CrossRef]
DOI: https://doi.org/10.7199/ped.oncall.2021.28
|
| Cite this article as: | | Melo B, Araújo R, Azevedo M J, Amorim R, Palhau L. Management of sialorrhea in children. Pediatr Oncall J. 2021;18: 71-76. doi: 10.7199/ped.oncall.2021.28 |
|