
Introduction
Haemophilus influenzae type b infection is one of the most common causes of invasive bacterial infection in <5 yr old children. Mass vaccination against Hib led to the virtual elimination of the disease from such countries like Finland, the UK, the USA, etc1,2. In developing countries like India, it is still a major cause of meningitis & pneumonitis as these countries can not afford mass vaccination programs. There is an urgent need to establish the disease burden in such countries so that Govt. sponsored mass vaccination programs can be started to decrease the disease burden due to Hib. This will help to eliminate or even eradicate Hib from the world.
Epidemiology
95% of invasive disease due to H. influenza is caused by type b & 59% by type a or non-typeable H. influenza. 95% of Hib occur in children <5 yrs old. More is the % of the population of children <5 yrs, more will be the number of cases of Hib per year in that country. Developing countries have both more incidence of Hib/100,000 in <5 yr old children & more % of the population <5 yr old. This leads to a heavy disease burden on the otherwise fragile health care system. Male to female ratio in some studies are shown to be 2:1 in UK & 3:2 in India.5
It is estimated that 3 million cases of Hib infection occur every year world over and 0.375 million of them die due to Hib. Studies from the west have indicated an annual incidence of 50-100/100000 in children <5 yrs of age as shown in Table 12,6. 50-60% of total Hib presents as meningitis, 5-8% each as pneumonitis, epiglottitis, skin infections, bone infections, bacteremia & others. Whereas data from developing countries like India is meager, the incidence varies from 100-250/100,000 in children <5 yrs. Majority of the present as meningitis (70-90%) or pneumonitis (10-20%). Epiglottitis & skin infection of the face virtually do not occur in developing countries.2,6
95% of invasive Hib disease occurs in <5 yrs old children. The peak occurs later in developed countries e.g. in Finland 28% of cases occur in <1 yr old & 40% in <18 months old children. In UK, 44% occurs in <1 year old and 71% is <2 yr old children, in USA 38% occurs in <1 yr & 60% occur in <18 months old children, in Netherlands 37% occurs in <1 yr old2,6. This means that the peak occurs at 9-18 months of age in such countries. This leads to fewer cases of meningitis (50-60%) & pneumonitis (<9% of total) whereas epiglottitis & facial skin infections occur in these countries. This is because the older the child, the more is the natural immunity & less is the severity of the disease. Whereas in developing countries the peak occur early at 6-9 months e.g. in Gambia 84% of cases occur in <1 yr (49% <6 months), in Senegal 69% occur <1 yr (32% in <6 months) & in Thailand 76% occur <1 yr (50% in <6 months)2,6. This leads to severe invasive disease as natural immunity is lacking in such young infants. This leads to more chances of meningitis (70-90%) & pneumonitis (10-20%) & virtual absence of epiglottitis & facial skin infections. It is interesting to note that epiglottitis & facial skin infections appear in such countries when the disease burden is reduced due to mass vaccination which leads to shifting to older age for the peak. Even within countries, there may be a difference in incidence depending upon the economical & socio-cultural differences e.g. in Nerago USA, the annual incidence is up to <50/100,000 <5 yr with 81% of cases occurring in <1 yr old or Alaska where the incidence could be as high as <50/100000 <5 yr with 67% of cases occurring in <1 yr old children.2,6
Table I: Incidence of Hib in various countries
| Country | Total Hib / 100,000<5 yr |
Hib meningitis/ 100,000<5 yr |
Mass immunization Year |
% drop / Year |
| Finland |
53 |
16-43 |
1988-89 |
100%/1991 |
| Netherlands |
>60 |
22 |
1993 |
85%/1994 |
| U. K. |
60-120 |
25 |
1992 |
98%/1994 |
| U. S. A. |
112 |
60 |
1989-93 |
99%/1995 |
| Iceland |
63 |
43 |
1989 |
100%/1991 |
| Alaska |
450 |
280-450 |
- |
- |
| Gambia |
60-270 |
60-70 |
- |
- |
| Australia |
59 |
25 |
1993 |
70%/1995 |
| Chile |
50-70 |
25 |
- |
- |
| Israel |
35 |
18 |
- |
- |
| India |
80-100 |
50-60 |
- |
- |
In India, only hospital-based data is available as shown in Table II. It shows that 30-45% of cases of meningitis & 8-12% of cases of pneumonitis are due to Hib4,7. These values are similar to those seen in European countries before mass vaccination suggesting similar epidemiology. It is estimated that the annual incidence of Hib in India is 50-60/100000 in <5 years4. The IBIS study has shown that 76% occurs in <1 yr with a peak at 6-9 months (range <1 month to >9 yr old individual)8.
Table II: Hospital-based data on Hib meningitis in India
| City/year | Hib% | Pneumococcus% | Meningococcus % |
| Delhi (1981) | 25 | 45 | 6 |
| Chennai(1994) | 28 | 21 | 4 |
| Vellore (1985) | 38 | 33 | 2 |
| Mean | 31 | 29 | 4 |
| Of culture (%) | (45) | (43) | (5) |
| Pondicherry(1990) | 8 | 30 | ? |
HIB - Clinical Spectrum
Hib leads to a spectrum of clinical manifestations starting from asymptomatic carrier state to various types of invasive diseases including meningitis, pneumonia, epiglottitis, bacteremia, skin infections, bone infection & others.
Carrier State
60-80% of healthy people are carriers of all types of H. influenza in their upper respiratory tract. Of this Hib is a commensal in children <5 yrs. In the west, 3-5% of children are carriers whereas in developing countries it rises to 15-30% of children9. It is rare before 6 months, peaks at 2-5 years & then falls >5 yrs of age. In the west, the carrier state remains for 5-6 weeks whereas in developing countries it can remain for months. Similarly, age at 1st exposure is early in developing countries & later in western worlds.
Hib gets transmitted from a carrier to a susceptible host via respiratory droplets let out on coughing or sneezing. When it invades the respiratory mucosa to enter into the bloodstream it leads to invasive disease.
Meningitis
Pyogenic meningitis is a leading cause of death due to Hib in children <5 years old. It can also lead to complications like ventriculitis, subdural effusion, or long-term sequelae in survivors.
Hib is a fastidious organism to grow on culture & hence one should use enriched media to increase the yield. Similarly, the use of antibiotics leads to decreased chances of growing causative organisms. In the pre-antibiotic era, 85% of cultures of CSF used to be positive in pyogenic meningitis, now it is positive in <50% of cases due to the use of antibiotics prior to collections of CSF. CIE for Hib & pneumococcus can increase the yield to 89% in such cases.4
In the west 50-60% of total Hib cases present as meningitis whereas it is 70-90% in developing countries due to early peak as discussed before. The annual incidence of Hib meningitis depends on the age of exposure. In the west where the peak occurs late 50-60% of Hib present as meningitis like in Finland, UK or USA whereas 70-90% of total Hib presents as meningitis in countries like Alaska, Gambia, etc.2,6 In India, the IBIS study has shown that 68% of all Hib cases presented as meningitis & 30% of all cases of pyogenic meningitis were due to Hib8. Other studies done in India has shown that 30% of total cases of pyogenic meningitis (45% of CSF culture +ve cases) were caused by Hib, 29% (43% of CSF culture-positive cases) were due to pneumococcus & 4% (59% of CSF culture-positive cases) were due to meningococcus & rest due to other organisms4,7.
Studies from the west have shown 3-5% mortality due to Hib meningitis in the UK, USA, or Finland. In developing countries, the mortality is as high as 30-50% due to a lack of a proper health care system2. With increasing resistance of Hib to antimicrobials, one has to use 3rd generation cephalosporins like cefotaxime or ceftriaxone as the first line of the drug. One study showed 100% mortality when cefotaxime was delayed in MDR Hib meningitis4,7. The IBIS study has shown 30% mortality due to Hib meningitis in <1 yr old & 10% in >1 year old. The mortality in non-meningitis Hib cases was also 10%8.
10-16% of survivors of Hib meningitis have long term sequelae in the west. This figure rises to 30-40% in developing countries.11% of survivors have deafness, 15% have language problems, 11% have mental retardation & 25% have motor defects or visual defects. This adds to the cost of treatment2,8.
Pneumonitis
Hib pneumonia is usually seen between 4 months to 4 years of age. 40% of them have associated bacteremia or meningitis. In the west, it is rare & is seen in 2-4% of total Hib cases whereas in developing countries 8-20% of total Hib cases present as pneumonia2. In some south-east Asian countries, it can account for up to 40% of total Hib cases2.
It is difficult to ascertain the causative organism in pneumonia as <10% of cases have positive blood cultures, <50% have positive lung tap culture. It is difficult to perform lung tap in all cases. Similarly, 50% of cases have positive CIE when done on blood or lung tap or pleural fluid.
One study from Calcutta has shown that 46% of lung tap cultures grew Hib4. Another study from Delhi has shown that of the cases of pneumonia 28% were caused by pneumococcus and 19% due to Hib. Of the Hib cases, 9% had positive blood culture & 10% more had positive CIE.11 A study from Vellore has shown that 8% of pneumonia cases had Hib grown on blood cultures, 30% had pneumococcus, 28% grew staphylococcus aureus & 40% grew other organisms4. 2 studies from Chandigarh have focused on respiratory diseases3,10. In one study, Ayyagiri et al showed that of acute upper respiratory tract disease 7% were due to Hib & 21% due to non-typable H. influenza; of acute lower respiratory tract disease 6% were caused by Hib & 9% by non-typeable H. influenza & none of the cases of chronic respiratory tract disease was caused by Hib. Similarly in another study, they showed that of pneumonia cases 8% were due to Hib & 61.5% due to pneumococcus & 20% due to staphylococcus. Lastly, the IBIS study has shown that of total Hib cases, 16% presented as pneumonia as proved by positive blood culture or pus culture8.
Others
8-12% of total Hib cases present as epiglottitis. It is usually seen in 2-4 yr old child & rarely in adults. The patient develops sudden onset of fever, drooling of saliva, anxious look, inflammation of the larynx, upper respiratory tract & epiglottis which becomes enlarged & red. Patients can die of choking. It is commonly seen in developed countries but virtually not seen in developed countries. In such countries, however, epiglottitis is seen pushing the peak age of Hib to older age2.
Similarly, skin infections involving the face is rarely seen in countries like India but was commonly seen in the west before mass vaccination.
Factors Affecting HIB Disease
Age is the most important factor affecting both incidence and type of Hib disease as discussed before. Males are affected often than females in a ratio of 2:1 to 3:2. Poor socioeconomic conditions, overcrowded housing, daycare center setup adversely affect the incidence of Hib disease. Certain diseases like asplenia, sickle cell anemia, splenectomy, immunodeficiency, nephrotic syndrome, complement deficiency, etc. also predispose to Hib disease. Chemical pollutants can increase the chances of carriage & invasion by Hib. Lastly, access to the health care system & vaccination will decide both the incidence & outcome of Hib disease.2
HIB Prevention
As discussed above, the burden of Hib disease is tremendous. 3 million children suffer from invasive Hib disease annually world-over, of which 0.37 million succumb to it. Of the survivors of Hib meningitis, 20-30% have long term sequelae. Increasing microbial resistance has forced us to use 3rd generation cephalosporins as 1st line dung adding to the cost of therapy. Poor health infrastructure in developing countries leads to increased mortality. Chemoprophylaxis will only succeed in blocking 29% of transmission which occurs from index case & is not a long-lasting solution.
Excellent conjugate Hib vaccines are available & used for mass vaccination since 1980. They have proved to be extremely potent, safe and efficacious. Mass vaccination has lead to the elimination of Hib disease from countries like Finland, UK, USA, etc. It is possible to eradicate Hib by universal immunization world over.
Natural immunity:
Transplacental maternal antibodies protect a newborn until 6-8 weeks. Acquired immunity develops in baby only after 18-24 months following subclinical infection as before 18-24 months the immune system of the baby is less developed. This leads to a susceptible period mainly during 3-18 months of life; the Hib disease peaks during this period. Hence Hib vaccine has to be effective during this period of 3-18 months for it to be meaningful2.
HIB Vaccines
PRP: The first Hib vaccines contained capsular PRP alone. Hib capsule is made up of repetitive limits of polyribitol ribitol polysaccharide. Anti PRP antibodies have been shown to be produced on exposure to Hib & are protective in nature.
Being a carbohydrate antigen, PRP is a poor immunogen as it can not stimulate T cells. Instead, it can only stimulate B cells. This leads to two problems. One, it is not effective in <18-24 months old children as B cell immunity is not well developed at that age and hence it can be given only for >18-24 months old children. Secondly, in the absence of T cell assistance, it can only induce IgM antibodies which are short-lasting & in low titres. Hence PRP failed to induce protection in the most vulnerable period i.e. <18 months of age & it induced short-lasting immunity. It also failed to reduce the carrier state.
Field studies done in Finland showed good efficacy of the PRP vaccine given at 24 months, as the peak of the disease occurs during that age in Finland. In other countries like the UK, the USA where the peak occurs early, PRP failed to demonstrate good efficacy given at 18-24 months of age2
Conjugated Hib vaccines:
2nd generation Hib vaccines are conjugated Hib vaccines. Here the PRP is conjugated with a carrier protein. The carrier protein sort of "fools" T cell & induces them to respond to the attached PRP. This makes PRP now T cell-dependent. The advantages of the conjugate vaccines are 3 folds. Firstly, it is effective from 6 weeks of age onwards. Secondly, it induces IgG antibodies & leads to boosting on repeated doses leading to better short term & long term immunity. Being T cell-dependent it stimulates memory T cells which will lead is anamnestic response even years later. Lastly, it also induces IgA antibodies which are secreted in nasal secretions. This will postpone & reduce the chances of carrier rate leading to herd immunity.
Types of conjugated Hib Vaccines:
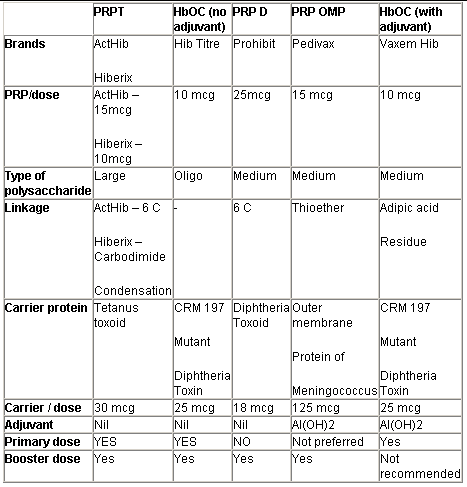
The conjugate Hib vaccines differ from one another depending on the size of the PRP molecule, the type of carrier protein, the type of linkage between PRP & carrier protein, and the presence or absence of an adjuvant in the vaccine as shown in table III.
PRP-T:
This vaccine has tetanus toxoid as the carrier protein which is attached to PRP via 6 carbon linkage or carbodiimide condensation. It contains 10-15 mcg of large PRP per dose & has 30 mcg of tetanus toxoid. It is an excellent & potent vaccine with proven field efficacy in many trials. It has no adjuvant. It is effective from 6 weeks of age onwards.
Table III: Types of Hib conjugate vaccine
Anti PRP antibody:
All the vaccines induce anti-PRP antibodies which are protective. In past, when PRP was the only vaccine available 2 levels of antibodies were taken as significant. Titres >0.15 mcg/ml were taken as protective for immediate but short-term period whereas titres >1.0 mcg/ml were taken as high titres which will protect for a long term up to 5 yrs of life. This is because PRP was effective only>18-24 months leading to poor titres & no boosting effect
Now with the availability of the conjugate vaccine, there is T cell response which leads to very high titres, IgG type of antibodies, and boosting effect due to T memory cells. Hence all such vaccines will naturally lead to long-term protection. Even if titres fall there will anamnestic response on exposure to Hib. Hence titres of >1.0 mcg/ml are probably irrelevant today. Yet more studies talk of cut off levels of >0.15 mcg/r ml & >1.0 mcg/ml while describing the immune response to the vaccine2.
Vaccine Efficacy
Hib conjugate vaccines are very immunogenic. Studies have shown excellent seroprotection and high antibody titres following vaccination with PRDT & HbOC vaccines. As discussed before PRP OMP is a good vaccine for first dose protection but not as immunogenic at the end of the schedule. PRP-O is the least immunogenic & hence not approved for use for primary dose but only as a booster dose.
98-100% of vaccines develop titres >0.15 mcg/ml at the end of 3 doses and GMT achieved is also high in the range of 4-6 mcg/ml. The antibody titres fall over the next 1 year. 90% still have titres >0.15 by 18-24 months. A booster given at 15-18 months leads to 100% of vaccinees reaches >0.15 mcg/ml level and GMT gets boosted by 60-70 times to a level of 60-90 mcg/ml.12
One of the studies on PRPT shown the kinetic of antibody development as seen in Table IV. One can see that 50% are protected by the first dose, 95% by the second dose, and 99% by 3rd dose of vaccines & 100% by the booster dose. The GMT at the end of 3 doses is 5.4 mcg/ml which falls to 1.22 mcg/ml by 18 months & reaches a high level of 84.7 mcg/ml after the booster dose.17
Table 4: Kinetics of anti PRP antibody following PRPT vaccine PRPT +DPT/IPV at 3, 4, 5 months
PRP OMP and HbOC vaccine with adjuvant lead to 80% of vaccinees achieving a titre of >0.15 mcg by first dose itself. After this, the titres for PRP OMP are not boosted with subsequent doses whereas HbOC with adjuvant vaccine shown similar titres & % seroprotection as PRPT or HbOC without adjuvant.
Similar results have been obtained in studies done on infants from developing countries like Gambia, Alaska, Chile, Philippines, India etc 18).
Comparison:
One study compared 4 vaccines viz. PRP D, PRP OMP, PRPT & HbOC vaccine for their immunogenicity as shown in table V. HbOC vaccine with adjuvant is recently available & data on this vaccine is shown in table VI.
Table V: Comparison of immunogenecity of various Hib vaccines16
| Anti PRP mcg/ml |
PRP OMP |
HbOC |
PRPT |
PRPD |
| Pre GMT |
0.11 |
0.07 |
0.10 |
0.07 |
| >1st GMT |
0.8 |
0.1 |
0.1 |
- |
| >2nd GMT |
0.8 |
0.3 |
0.3 |
0.08 |
| >3rd GMT |
1.14 |
3.08 |
3.64 |
0.28 |
| % > 0.15 |
92.0 |
93.0 |
99.0 |
58.0 |
| % > 1.0 |
55.0 |
75.0 |
83.0 |
28.0 |
Table VI: Kinetics of antibody after HbOC with adjuvant
| Anti PRP mcg/ml |
> 1st dose |
> 2nd dose |
> 3rd dose |
| % > 0.15 |
80 |
100 |
100 |
| % > 1.0 |
45 |
91 |
100 |
| GMT |
0.75 |
5.4 |
22.4 |
It shows that PRPT & HbOC vaccines are excellent vaccines with protection in 50% with the first dose and 95% of vaccinees with 2nd dose onwards. The % seroprotection & GMT are comparable though the titres are marginally better with PRPT than with HbOC in this study. PRP OMP was good as far as first dose protection which was achieved in the majority but the titres did not get boosted with subsequent doses. PRP D was the least immunogenic with <60% seroprotected after 3 doses.
HbOC with adjuvant vaccine shows protection in 80% with the first dose, 100% with the second dose & the 3rd dose acts as a booster. The titres at the end of 3rd dose were 22.4 mcg/ml with this vaccine. There is one study comparing HbOC without adjuvant with HbOC with adjuvant. It showed that 93% of infants achieved titres of >0.15 mcg/ml with both the vaccines & 7-8% & 6% of infants achieved titres of >1.0 mg/ml at the end of 3 doses with adjuvant vaccines & non-adjuvant vaccines respectively. This shows that both these vaccines are similar and excellent in efficacy.
Clinical efficacy:
There are innumerable reports of clinical efficacy of Hib vaccines in field trials. Oxford, UK study showed 100% efficacy of PRP vaccines given to infants (given 3 primary doses alone) over 2 years follow up2. Similar studies from Finland using PRP D vaccine in >0.1 million showed 90% efficacy in infants given 3 primary doses & 1 booster dose2. A study using HbOC in California showed 100% efficacy & in Finland 97% efficacy using 3 doses and a booster in infants2. PRP OMP vaccine showed 97% efficacy with the first dose itself in Arizona area2. Other studies done with PRPT have shown 91.7% efficacy in Chile & 100% efficacy in Finland. It is estimated that the first dose of vaccine gives 71%, 2nd dose 89% & 3rd dose 95%-100% clinical efficacy.
Effect on carrier state:
A study from Oxford, UK showed that there was a significantly low level of carriage in vaccinated infants compared to controls. The carrier rate was 1.5% in vaccinated as compared to 6.3% in unvaccinated infants. Amongst children with a family history of exposure to Hib the carrier rate was 8.7% in vaccinated & 38.5& in the unvaccinated group. Only 3.7% of vaccinated siblings were the carrier of an index carrier as compared to 12.0% in the unvaccinated group. There was no difference in the period for which a child remained carrier as it was 5.2 weeks in vaccinated Vs 5.6 weeks in the unvaccinated group9. A similar study from Finland has shown that 3.5% of unvaccinated children were carrier as compared to 0% of vaccinated children2. In Iceland, it was shown that the carrier rate dropped from 16% to 0.5% in 2 years after the mass vaccine.
The decrease in carrier rate can explain herd immunity as seen in countries resorting to mass vaccination.
Availability:
All conjugate vaccines are available as 0.5 ml volume per dose. It is either ready to use liquid (HbOC) or in the lyophilized form (rest of the vaccines) with the diluent provided separately. It is either available as a single dose or as multi-dose (10 doses) vial. HbOC with the adjuvant vaccine has adjuvant available separately which has to be mixed with the vaccine just before vaccinating (adjuvant mixed with the vaccine is not stable & hence is provided separately). The cost of a unit dose is Rs 350 to 400 per dose.
Route:
Hib vaccines are given intramuscularly. In cases of a severe bleeding disorder, it can be given subcutaneously if IM injection is contraindicated. If given IM in such cases good pressure should be applied at the injection site for 5 minutes & it should be preferably given after factor replacement in cases of factor deficiency2. It should not be given IV or intradermally.
Schedule:
For all the vaccines the schedule depends on the age. For a child less than 6 months, 3 primary doses are given at 1-2 months interval starting from 6-8 weeks of age. It can be given along with other EPI vaccines on the same day using separate sites & separate syringes. For a child between 6-12 months, 2 primary doses are given at 6-8 weeks interval. For 12-15 months old child only one primary dose is given. All these children are given a booster dose at 15-18 months of age. Again the booster can be given along with MMR or booster of OPV/DPT. If the child is >15 months old he is given only one dose i.e. directly the booster.
PRP OMP is given as 2 primary doses at 2 months interval followed by a booster at 1 year of age. HbOC vaccine with adjuvant is given as 3 dose schedule below 1 year at 4-8 weeks interval (the 3rd dose acts as a booster) & as 2 dose schedule at 4-8 weeks interval for >1-year-old child (the 2nd dose acts as a booster)15.
Impact of Mass Vaccination
Various countries took to mass vaccination using the conjugate Hib vaccine since 1985-1990. They all have shown more than 95-99% reduction in clinical cases due to Hib just in 2-5 years of such vaccination programme. In some cases, the benefits were also seen in the unvaccinated minority due to the herd immunity effect.
In the USA between 1985-87, there was no impact on the use of PRP at 18-24 months of age. Between 1987-1990, PRPD was used as 3 primary & 1 booster dose which showed an 80% drop in incidence. After 1990, HbOC & PRPT are extensively used. Even with 60% coverage, there was a 90-95% drop in incidence2. Similarly, Finland resorted to mass vaccination in 1988 using initially PRP OMP or HbOC & later HbOC or PRPT. It showed 87% drop with PRP OMP, 95% drop with HbOC & 95-98% drop with PRPT at coverage of 90-95%2,19. In UK mass vaccination began in 1992 which showed 99% drop in <1-year-old children, 97% drop in 1-2 years old & 94.7% drop in 2-3 years old children at 92% coverage2,20.
Hence it is possible to eliminate or even eradicate Hib by mass vaccination program.
Cost-effectiveness of mass vaccination:
When considering the cost-effectiveness of mass vaccination programmes by any country one has to take into consideration on one hand the cost of the vaccine, the cost of administering versus the cost of diagnosis, treatment & management of sequelae of Hib disease. This cost will differ from country to country.
The studies from the USA have shown that it is cost-effective to mass vaccinate with Hib vaccine2,21. Similar studies from developing countries need to be done depending on local factors & priorities so that decisions on government sponsored universal immunization can be undertaken. In some countries, it is estimated the vaccine will be cost-effective only if each dose of vaccine costs less than one US dollar which is still not possible as the vaccines are very costly.
Interchangeability of Vaccines
Primary doses:
It is ideal to finish the schedule of primary doses with the same brand. In case it is not known one can use any other vaccine. Studies have shown that the immune response is the same. PRP D is not used for primary doses. Some studies have used PRP OMP as 1st dose to get maximum 1st dose protection followed by 2nd & 3rd dose with either HbOC or PRPT to get maximum boosting titres.22
HbOC with the adjuvant vaccine has a different schedule as compared to the other 4 vaccines. Hence it should not be interchanged for other vaccines. No such studies are even available.
Booster dose:
Again it is good practice to use the same brand for a booster as that used for primary doses. However, all the 4 vaccines can be interchanged for booster. PRP D is licensed to use a booster (2.13). HbOC with adjuvant has a different schedule & hence should not be interchanged with other vaccines.
Why do we need a booster?
As seen before 98-99% achieved a titre of >0.15 mcg/ml & 90% of them have >1.0 mcg/ml level with GMT of 5-6 mcg/ml at the end of 3 doses. 90% maintain the level >0.15 mcg/ml till 15-18 months but the titre falls to 1.22 mcg/ml by that time. Hence a booster is given at 15-18 months which will lead to 100% achieve a titre >1.0 mcg/ml with GMT of 40-60 mcg/ml17. This will protect the children for a long time, certainly beyond 5 years of age.
Side Effects of The HIB Vaccines
Hib conjugate vaccines are one of the safest vaccines proved in many studies.
Local:
3-5% vaccinees develop local pain, 10% develop redness, 2-4% develop swelling. These are mild in nature & lasts for 1-2 days. One can use paracetamol for pain2,24.
Systemic:
10-15% develop fever which is mild, lasts for 1-2 days & responds to paracetamol. Other side effects include loss of appetite in 15-20%, restlessness in 15-20%, excessive crying in 20-22%, vomiting in 7-10% & diarrhoea in 10-15% of cases. Again these symptoms are mild and self-limiting2,24.
All the local & systemic side effects are more common with primary doses than booster dose.
HIB Combination Vaccines
OPV/IPV, DPT, Hep B & Hib vaccines need to be given at about the same age of 6-8weeks onwards. To be meaningful, the schedule of all these vaccines has to be completed in time. This means giving these vaccines on the same day.
One can give more than 1 vaccine either separately using a separate syringe at the separate site or use a combination vaccine containing more than 1 antigen in the same vaccine. Giving vaccines separately on the same day will mean multiple pricks & the use of multiple syringes. It is less acceptable by parents at times whereas combination vaccines mean a single prick for multiple antigens, use of only one syringe which will save on cost & also easy storage in the fridge as one will need to keep less number of units. Combination vaccines also save on several visits than when given on separate days.
Hib vaccine has been combined with DPT (quadrivalent), DPaT (quadrivalent), DPT/IPV (pentavalent), DPaT/IPV (pentavalent), DPT/HepB, DPT/IPV/HepB (hexavalent). The studies show a similar immune response to all the components of vaccines than when given separately. Some studies have used Hib & these other vaccines given separately at different sites but on the same day whereas others have mixed Hib vaccines with these vaccines. Some studies have shown interference with anti PRP titre & anti pertussis titres by mixing vaccines. Some have shown higher anti-PRP, anti diphtheria & antitetanus titres by mixing the vaccines in the same syringe due to the priming effect of carrier protein. Hence one should not mix Hib with another vaccine in the same syringe unless recommended by manufacturers based on studies.
HIB-mortality
Meningitis & epiglottitis are 2 common causes of mortality. In the west, the mortality is low & is 3-5%. In developing countries, it is as high as 30-50% due to suboptimal health care system & access2,4,7. It is estimated that world over 3,75,000 children die due to Hib every year. In India, the IBIS study has shown that the mortality due to meningitis is 30% in <1 yr old children & 10% in >1 yr old. Similarly, mortality due to non-meningitis Hib cases is 10%8. The mortality is likely to increase due to increasing drug resistance.
HIB Drug Resistance
Of late there is an increase in the incidence of drug resistance in Hib. Ampicillin resistance was reported in 1974, chloramphenicol resistance in 1978. Both ampicillin and chloramphenicol resistant strains were reported in 1980 from the UK, USA, Bangkok & Spain12. Since then multidrug resistance mediated by R plasmid has spread all over the world posing therapeutic challenges, threatening to increase mortality & increasing the cost of treatment.
1st multidrug-resistant strain causing meningitis in India was reported from Chandigarh which proved fatal12. This was followed by 2 more cases reported from Pondicherry & one more case from Chandigarh in 199013. Use of cefotaxime saved this child. A large study from Vellore in 1992 reported that 42.5% of total Hib cases were MDR strains with 80% resistant to ampicillin, 90% to chloramphenicol & sulpha drugs & none to cefotaxime4,7. This prompted them to use 3rd generation cephalosporins as the first-line drug in cases of meningitis as mortality was 100% in MDR cases if cefotaxime was delayed. This is in contrast to the UK where between 1985-90 only 12.5% strains were resistant to ampicillin & none to chloramphenicol. The increased incidence of MDR in India was be related to the widespread misuse of antibiotics in the general population. A similar report from Nagpur in 1996 showed that 80% of Hib infants were resistant to both ampicillin and chloramphenicol14. In 1996 the second report from Vellore showed 30% resistance to ampicillin, 17% to chloramphenicol, and 15% to both these drugs4,7. Lastly, the IBIS study has shown 56% resistance to chloramphenicol, 40% to ampicillin & none to 3rd generation cephalosporin8.